Abstract
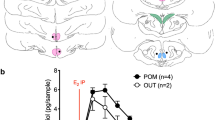
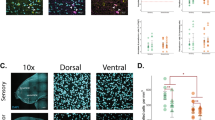
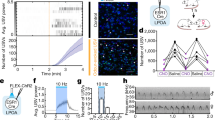
Neurosteroids are powerful modulators of brain function and behavior, yet their dynamics in the brain have remained elusive. Using in vivo microdialysis in male zebra finches, we found that local estradiol levels increased rapidly in the forebrain during social interactions with females. Furthermore, when males were exposed to other males' songs, local estradiol levels also increased and testosterone levels dropped in a cortical/pallial auditory region that is analogous to mammalian auditory cortex. We also found that local estradiol and testosterone levels were differentially regulated in this same region by the conventional neurotransmitters glutamate and GABA, respectively. This study provides direct evidence that forebrain steroid levels are acutely and differentially regulated during social behavior in a region-specific manner and in a rapid time course similar to that of traditional neuromodulators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Boehning, D. & Snyder, S.H. Novel neural modulators. Annu. Rev. Neurosci. 26, 105–131 (2003).
Gibbs, T.T., Russek, S.J. & Farb, D.H. Sulfated steroids as endogenous neuromodulators. Pharmacol. Biochem. Behav. 84, 555–567 (2006).
Baulieu, E.E. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 23, 963–987 (1998).
Mathieu, M. et al. Immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase and 5 alpha-reductase in the brain of the African lungfish Protopterus annectens. J. Comp. Neurol. 438, 123–135 (2001).
Stoffel-Wagner, B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann. N Y Acad. Sci. 1007, 64–78 (2003).
Tsutsui, K., Matsunaga, M., Miyabara, H. & Ukena, K. Neurosteroid biosynthesis in the quail brain: a review. J. Exp. Zoolog. A Comp. Exp. Biol. 305, 733–742 (2006).
Balthazart, J. & Ball, G.F. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 29, 241–249 (2006).
Woolley, C.S. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 47, 657–680 (2007).
Lambert, J.J., Belelli, D., Peden, D.R., Vardy, A.W. & Peters, J.A. Neurosteroid modulation of GABA(A) receptors. Prog. Neurobiol. 71, 67–80 (2003).
Remage-Healey, L. & Bass, A.H. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 1126, 27–35 (2006).
Cornil, C.A., Ball, G.F. & Balthazart, J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 1126, 2–26 (2006).
Holloway, C.C. & Clayton, D.E. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat. Neurosci. 4, 170–175 (2001).
Hojo, Y. et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017 alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 101, 865–870 (2004).
Mukai, H. et al. Local neurosteroid production in the hippocampus: Influence on synaptic plasticity of memory. Neuroendocrinology 84, 255–263 (2006).
Schlinger, B.A. & Brenowitz, E.A. Neural and hormonal control of birdsong. in Hormones, Brain and Behavior (eds. Arnold, A.P., Etgen, A.M., Fahrbach, S.E., Rubin, R.T. & Pfaff, D.W.) 799–838 (Academic Press, San Diego, California, 2002).
Bottjer, S.W. & Johnson, F. Circuits, hormones and learning: vocal behavior in songbirds. J. Neurobiol. 33, 602–618 (1997).
London, S.E., Monks, D.A., Wade, J. & Schlinger, B.A. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology 147, 5975–5987 (2006).
Soma, K.K., Alday, N.A., Hau, M. & Schlinger, B.A. Dehydroepiandrosterone metabolism by 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology 145, 1668–1677 (2004).
Peterson, R.S., Yarram, L., Schlinger, B.A. & Saldanha, C.J. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc. Biol. Sci. 272, 2089–2096 (2005).
Schlinger, B.A. & Arnold, A.P. Brain is the major site of estrogen synthesis in a male songbird. Proc. Natl. Acad. Sci. USA 88, 4191–4194 (1991).
Saldanha, C.J. et al. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 423, 619–630 (2000).
Bolhuis, J.J. & Gahr, M. Neural mechanisms of birdsong memory. Nat. Rev. Neurosci. 7, 347–357 (2006).
London, S.E. & Clayton, D.F. Functional identification of sensory mechanisms required for developmental song learning. Nat. Neurosci. 11, 579–586 (2008).
Peterson, R.S., Saldanha, C.J. & Schlinger, B.A. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata). J. Neuroendocrinol. 13, 317–323 (2001).
Amateau, S.K., Alt, J.J., Stamps, C.L. & McCarthy, M.M. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology 145, 2906–2917 (2004).
Flores, F., Naftolin, F., Ryan, K.J. & White, R.J. Estrogen formation by isolated perfused rhesus monkey brain. Science 180, 1074–1075 (1973).
Barbaccia, M.L. et al. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol. Biochem. Behav. 54, 205–210 (1996).
Bixo, M., Backstrom, T., Winblad, B. & Andersson, A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J. Steroid Biochem. Mol. Biol. 55, 297–303 (1995).
Reddy, D.S. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3 alpha-androstanediol and 17 beta-estradiol. Neuroscience 129, 195–207 (2004).
Remage-Healey, L. & Bass, A.H. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 27, 1114–1122 (2007).
Livingston, F.S., White, S.A. & Mooney, R. Slow NMDA-EPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat. Neurosci. 3, 482–488 (2000).
Agis-Balboa, R.C. et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 103, 14602–14607 (2006).
London, S.E., Boulter, J. & Schlinger, B.A. Cloning of the zebra finch androgen synthetic enzyme CYP17: A study of its neural expression throughout posthatch development. J. Comp. Neurol. 467, 496–508 (2003).
Pinaud, R., Fortes, A.F., Lovell, P. & Mello, C.V. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J. Neurobiol. 66, 182–195 (2006).
Hultcrantz, M., Simonoska, R. & Stenberg, A.E. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. (Stockh.) 126, 10–14 (2006).
Sisneros, J.A., Forlano, P.M., Deitcher, D.L. & Bass, A.H. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science 305, 404–407 (2004).
Saalmann, Y.B., Morgan, I.G. & Calford, M.B. Neurosteroids involved in regulating inhibition in the inferior colliculus. J. Neurophysiol. 96, 3064–3073 (2006).
Maney, D.L., Cho, E. & Goode, C.T. Estrogen-dependent selectivity of genomic responses to birdsong. Eur. J. Neurosci. 23, 1523–1529 (2006).
Yague, J.G. et al. Aromatase expression in the human temporal cortex. Neuroscience 138, 389–401 (2006).
Gobes, S.M.H. & Bolhuis, J.J. Birdsong memory: a neural dissociation between song recognition and production. Curr. Biol. 17, 789–793 (2007).
Frye, C.A., Duffy, C.K. & Walf, A.A. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 88, 208–216 (2007).
Packard, M.G. Post-training estrogen and memory modulation. Horm. Behav. 34, 126–139 (1998).
Oberlander, J.G., Schlinger, B.A., Clayton, N.S. & Saldanha, C.J. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm. Behav. 45, 250–258 (2004).
Marler, P., Peters, S., Ball, G.F., Dufty, A.M. & Wingfield, J.C. The role of sex steroids in the acquisition and production of birdsong. Nature 336, 770–772 (1988).
Meitzen, J., Moore, I.T., Lent, K., Brenowitz, E.A. & Perkel, D.J. Steroid hormones act trans-synaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J. Neurosci. 27, 12045–12057 (2007).
Murphy, N.P., Lam, H.A. & Maidment, N.T. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J. Neurochem. 79, 626–635 (2001).
Sasaki, A., Sotnikova, T.D., Gainetdinov, R.R. & Jarvis, E.D. Social context-dependent singing-regulated dopamine. J. Neurosci. 26, 9010–9014 (2006).
Mello, C.V., Velho, T.A.F. & Pinaud, R. Song-induced gene expression: a window on song auditory processing and perception. Ann. N Y Acad. Sci. 1016, 263–281 (2004).
Acknowledgements
H. Lam provided technical support for microdialysis. M. Konishi provided the sound-attenuation chambers. I. Teramitsu and J. Goodson demonstrated surgical techniques. A. Briedbach, K. Faull and the staff of the University of California Los Angeles Pasarow GC/MS Core Facility (support from the National Science Foundation, CHE 0078299) provided technical support. N. Tillakaratne provided an ELISA plate reader. S. Cho assisted with tissue processing. C.I.M. Healey provided comments on the manuscript. This work was supported by the US National Institute of Neurological Disorders and Stroke (National Research Service Award F32NS058009-01) and the US National Institute of Mental Health (061994).
Author information
Authors and Affiliations
Contributions
L.R.-H. conducted the experiments and wrote the manuscript. N.T.M. provided methodological expertise and contributed to manuscript preparation. B.A.S. supervised the project and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Results and Supplementary Methods (PDF 141 kb)
Rights and permissions
About this article
Cite this article
Remage-Healey, L., Maidment, N. & Schlinger, B. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11, 1327–1334 (2008). https://doi.org/10.1038/nn.2200
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2200
This article is cited by
-
Sex-specific differences in zebrafish brains
Biology of Sex Differences (2022)
-
Non-sensory Influences on Auditory Learning and Plasticity
Journal of the Association for Research in Otolaryngology (2022)
-
Rapid changes in brain estrogen concentration during male sexual behavior are site and stimulus specific
Scientific Reports (2021)
-
Neuroestrogen synthesis modifies neural representations of learned song without altering vocal imitation in developing songbirds
Scientific Reports (2020)
-
Acute neuroestrogen blockade attenuates song-induced immediate early gene expression in auditory regions of male and female zebra finches
Journal of Comparative Physiology A (2020)