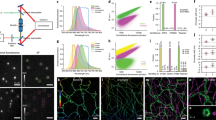
Abstract
We introduce far-red, fluorogenic probes that combine minimal cytotoxicity with excellent brightness and photostability for fluorescence imaging of actin and tubulin in living cells. Applied in stimulated emission depletion (STED) microscopy, they reveal the ninefold symmetry of the centrosome and the spatial organization of actin in the axon of cultured rat neurons with a resolution unprecedented for imaging cytoskeletal structures in living cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Barasoain, I., Díaz, J.F. & Andreu, J.M. Methods Cell Biol. 95, 353–372 (2010).
Huang, Z.J., Haugland, R.P. & You, W.M. Anal. Biochem. 200, 199–204 (1992).
Wulf, E., Deboben, A., Bautz, F.A., Faulstich, H. & Wieland, T. Proc. Natl. Acad. Sci. USA 76, 4498–4502 (1979).
Díaz, J.F., Barasoain, I., Souto, A.A., Amat-Guerri, F. & Andreu, J.M. J. Biol. Chem. 280, 3928–3937 (2005).
Lukinavičius, G. et al. Nat. Chem. 5, 132–139 (2013).
Dubois, J. et al. Bioorg. Med. Chem. 3, 1357–1368 (1995).
Milroy, L.G. et al. J. Am. Chem. Soc. 134, 8480–8486 (2012).
Bellamy, W.T. Annu. Rev. Pharmacol. Toxicol. 36, 161–183 (1996).
Cortes, J.E. & Pazdur, R. J. Clin. Oncol. 13, 2643–2655 (1995).
Bubb, M.R., Senderowicz, A.M., Sausville, E.A., Duncan, K.L. & Korn, E.D. J. Biol. Chem. 269, 14869–14871 (1994).
Steigemann, P. et al. Cell 136, 473–484 (2009).
Schneckenburger, H. et al. J. Microsc. 245, 311–318 (2012).
Gustafsson, M.G. J. Microsc. 198, 82–87 (2000).
Klar, T.A., Jakobs, S., Dyba, M., Egner, A. & Hell, S.W. Proc. Natl. Acad. Sci. USA 97, 8206–8210 (2000).
Hell, S.W. & Wichmann, J. Opt. Lett. 19, 780–782 (1994).
Paintrand, M., Moudjou, M., Delacroix, H. & Bornens, M. J. Struct. Biol. 108, 107–128 (1992).
Kitagawa, D. et al. Cell 144, 364–375 (2011).
Xu, K., Zhong, G. & Zhuang, X. Science 339, 452–456 (2013).
Riedl, J. et al. Nat. Methods 5, 605–607 (2008).
Bonne, D., Heuséle, C., Simon, C. & Pantaloni, D. J. Biol. Chem. 260, 2819–2825 (1985).
Cooper, J.A., Walker, S.B. & Pollard, T.D. J. Muscle Res. Cell Motil. 4, 253–262 (1983).
Viswanadhan, V.N., Ghose, A.K., Revankar, G.R. & Robins, R.K. J. Chem. Inf. Comput. Sci. 29, 163–172 (1989).
Byers, T.J. & Branton, D. Proc. Natl. Acad. Sci. USA 82, 6153–6157 (1985).
Behnke, O. J. Ultrastruct. Res. 31, 61–75 (1970).
Urban, N.T., Willig, K.I., Hell, S.W. & Nägerl, U.V. Biophys. J. 101, 1277–1284 (2011).
Held, M. et al. Nat. Methods 7, 747–754 (2010).
Göttfert, F. et al. Biophys. J. 105, L01–L03 (2013).
Schindelin, J. et al. Nat. Methods 9, 676–682 (2012).
Acknowledgements
The authors are grateful to J.E. Bear (University of North Carolina at Chapel Hill), P. Gönczy (École Polytechnique Fédérale de Lausanne (EPFL)), R. Jahn (University of Göttingen), D. Abankwa (Åbo Akademi University), U. Ruegg (University of Geneva) and A. Seitz (EPFL) for sharing reagents and for technical assistance. K.J. acknowledges support from the Swiss National Science Foundation and the National Centre of Competence of Research (NCCR) Chemical Biology. Support from the Körber foundation was received through the European Science Prize to S.W.H. D.W.G. acknowledges support from the European Community's Seventh Framework Programme FP7/2007-2013 under grant agreements nos. 241548 (MitoSys) and 258068 (Systems Microscopy) and from an European Research Council (ERC) Starting Grant (agreement no. 281198).
Author information
Authors and Affiliations
Contributions
G.L., L.R. and K.J. devised this study. All authors except S.R. and A.G. contributed to manuscript writing. G.L., E.D., A.M., C.B., C.S. and D.W.G. characterized the probes. L.R., A.G. and S.R. performed synthesis of probes, supervised by K.J., H.W. and H.-D.A.; G.L., E.D., F.G. and H.T. performed STED microscopy, guided by S.W.H.; G.L. and M.F. performed SIM microscopy.
Corresponding authors
Ethics declarations
Competing interests
G.L. and K.J. have filed a patent application on SiR derivatives (PCT/EP2011/064750). S.R., H.-D.A. and H.W. have filed a patent application on the use of jasplakinolide derivatives (PCT/EP2012/073002).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14, Supplementary Tables 1–3 and Supplementary Notes 1–3 (PDF 7301 kb)
Long-term live-cell microscopy of untreated control HeLa Kyoto cells stably expressing H2B-mRFP and MyrPalm-mEGFP.
The movie shows a region-of-interest of 1/16th of the full image frame and the full movie (0 – 23 h). (MOV 4300 kb)
Long-term live-cell microscopy of HeLa Kyoto cells stably expressing H2B-mRFP and MyrPalm-mEGFP, treated with 100 nM SiR-tubulin.
The movie shows a region-of-interest of 1/16th of the full image frame and the full movie (0 – 23 h), starting 0.5 h after addition of SiR-tubulin. (MOV 10798 kb)
Long-term live-cell microscopy of HeLa Kyoto cells stably expressing H2B-mRFP and MyrPalm-mEGFP, treated with 100 nM SiR-actin.
The movie shows a region-of-interest of 1/16th of the full image frame and the full movie (0 – 23 h), starting 0.5 h after addition of SiR-actin. (MOV 6974 kb)
Long-term live-cell microscopy of HeLa Kyoto cells stably expressing H2B-mRFP and MyrPalm-mEGFP, treated with 1 nM taxol.
The movie shows a region-of-interest of 1/16th of the full image frame and the full movie (0 – 23 h), starting 0.5 h after addition of taxol. (MOV 4344 kb)
Long-term live-cell microscopy of HeLa Kyoto cells stably expressing H2B-mRFP and MyrPalm-mEGFP, treated with 100 nM jasplakinolide.
The movie shows a region-of-interest of 1/16th of the full image frame and the full movie (0 – 23 h), starting 0.5 h after addition of jasplakinolide. (MOV 3735 kb)
2D SIM time series of microtubule dynamics in human primary dermal fibroblasts.
Cells were stained with 2 μM SiR-tubulin in complete DMEM medium for 60 min at 37°C and washed before imaging on a Nikon Eclipse Ti microscope. Time is indicated in min : s format. (AVI 10440 kb)
3D SIM time series of actin dynamics in lamellipodia of human primary dermal fibroblasts.
Cells were stained with 2 μM SiR-actin in complete DMEM medium for 120 min and imaged directly without washing on Nikon Eclipse Ti microscope. Time is indicated in min : s format. (AVI 12885 kb)
STED time series imaging of microtubule dynamics in human primary dermal fibroblasts.
Cells were stained with 2 μM SiR-tubulin in complete DMEM medium for 60 min at 37°C and washed before imaging on a STED microscope. Time is indicated in min : s format. (AVI 5124 kb)
Source data
Rights and permissions
About this article
Cite this article
Lukinavičius, G., Reymond, L., D'Este, E. et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods 11, 731–733 (2014). https://doi.org/10.1038/nmeth.2972
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2972
This article is cited by
-
Oligodendrocyte calcium signaling promotes actin-dependent myelin sheath extension
Nature Communications (2024)
-
A general strategy to develop fluorogenic polymethine dyes for bioimaging
Nature Chemistry (2024)
-
Resolution enhancement with a task-assisted GAN to guide optical nanoscopy image analysis and acquisition
Nature Machine Intelligence (2023)
-
Repair of airway epithelia requires metabolic rewiring towards fatty acid oxidation
Nature Communications (2023)
-
Annexin A1 is a polarity cue that directs mitotic spindle orientation during mammalian epithelial morphogenesis
Nature Communications (2023)