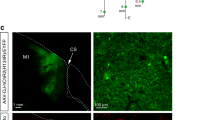
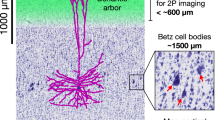
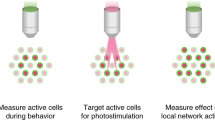
Abstract
Traditionally, mapping the motor cortex requires electrodes to stimulate the brain and define motor output pathways. Although effective, electrode-based methods are labor-intensive, potentially damaging to the cortex and can have off-target effects. As an alternative method of motor mapping, we photostimulated transgenic mice expressing the light-sensitive ion channel channelrhodopsin-2 in predominantly layer-5 output cortical neurons. We report that optical stimulation of these neurons in vivo using a stage scanning laser system resulted in muscle excitation within 10–20 ms, which can be recorded using implanted electromyogram electrodes or by a noninvasive motion sensor. This approach allowed us to make highly reproducible automated maps of the mouse forelimb and hindlimb motor cortex much faster than with previous methods. We anticipate that the approach will facilitate the study of changes in the location and properties of motor maps after skilled training or damage to the nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Penfield, W. & Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. J. Neurol. 60, 389–443 (1937).
Donoghue, J.P. & Sanes, J.N. Peripheral-nerve injury in developing rats reorganizes representation pattern in motor cortex. Proc. Natl. Acad. Sci. USA 84, 1123–1126 (1987).
Hosp, J.A. et al. Thin-film epidural microelectrode arrays for somatosensory and motor cortex mapping in rat. J. Neurosci. Methods. 172, 255–262 (2008).
Siebner, H.R. & Rothwell, J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp. Brain Res. 148, 1–16 (2003).
Shepherd, G.M.G., Pologruto, T.A. & Svoboda, K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38, 277–289 (2003).
Luo, L., Callaway, E.M. & Svoboda, K. Genetic dissection of neural circuits. Neuron 57, 634–660 (2008).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008).
Zhang, F., Aravanis, A.M., Adamantidis, A., de Lecea, L. & Deisseroth, K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 8, 577–581 (2007).
Nagel, G. et al. Channelrhodopsins: directly light-gated cation channels. Biochem. Soc. Trans. 33, 863–866 (2005).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Callaway, E.M. & Katz, L.C. Photostimulation using caged glutamate reveals functional circuitry in living brain-slices. Proc. Natl. Acad. Sci. USA 90, 7661–7665 (1993).
Feng, G.P. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Rho, M.J., Lavoie, S. & Drew, T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J. Neurophysiol. 81, 2297–2315 (1999).
Murphy, T.H., Li, P., Betts, K. & Liu, R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J. Neurosci. 28, 1756–1772 (2008).
Ferezou, I. et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56, 907–923 (2007).
Raineteau, O. & Schwab, M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 (2001).
Brown, C.E., Li, P., Boyd, J.D., Delaney, K.R. & Murphy, T.H. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 27, 4101–4109 (2007).
Winship, I.R. & Murphy, T.H. In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J. Neurosci. 28, 6592–6606 (2008).
Trachtenberg, J.T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Xu, H.T., Pan, F., Yang, G. & Gan, W.B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10, 549–551 (2007).
Kleim, J.A. et al. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron 40, 167–176 (2003).
Teskey, G.C., Monfils, M.H., VandenBerg, P.M. & Kleim, J.A. Motor map expansion following repeated cortical and limbic seizures is related to synaptic potentiation. Cereb. Cortex. 12, 98–105 (2002).
Franklin, K.B.J. & Paxinos, G. The mouse brain. In Stereotaxic Coordinates (Academic Press, New York, 1997).
Herfst, L.J. & Brecht, M. Whisker movements evoked by stimulation of single motor neurons in the facial nucleus of the rat. J. Neurophysiol. 99, 2821–2832 (2008).
Hall, R.D. & Lindholm, E.P. Organization of motor and somatosensory neocortex in albino rat. Brain Res. 66, 23–38 (1974).
Sievert, C.F. & Neafsey, E.J. A chronic unit study of the sensory properties of neurons in the forelimb areas of rat sensorimotor cortex. Brain Res. 381, 15–23 (1986).
Zhang, F. et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11, 631–633 (2008).
Graziano, M.S.A., Cooke, D.F., Taylor, C.S.R. & Moore, T. Distribution of hand location in monkeys during spontaneous behavior. Exp. Brain Res. 155, 30–36 (2004).
Acknowledgements
This work was supported by operating grants to T.H.M. (MOP-12675) and the In Vivo Imaging Centre from the Canadian Institutes of Health Research. We thank P. Wang, H. Erb, C. Jiang, A. Tung and N. Scott for assistance.
Author information
Authors and Affiliations
Contributions
O.G.S.A. and T.C.H. collected and analyzed data, and prepared the manuscript. J.D.B. developed software, analyzed data and performed histological analyses. A.G. performed optical design and fabrication of the device. T.H.M. conceived the work, analyzed data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
O.G.S.A., T.C.H., J.D.B. and T.H.M. are listed as inventors on a US patent application that covers aspects of the work described in the manuscript.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–2, Supplementary Methods (PDF 2031 kb)
Rights and permissions
About this article
Cite this article
Ayling, O., Harrison, T., Boyd, J. et al. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods 6, 219–224 (2009). https://doi.org/10.1038/nmeth.1303
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1303
This article is cited by
-
Cortical processing of flexible and context-dependent sensorimotor sequences
Nature (2022)
-
Brain motor and fear circuits regulate leukocytes during acute stress
Nature (2022)
-
Delayed motor learning in a 16p11.2 deletion mouse model of autism is rescued by locus coeruleus activation
Nature Neuroscience (2021)
-
Recent advances in development of devices and probes for sensing and imaging in the brain
Science China Chemistry (2021)
-
Optoacoustic brain stimulation at submillimeter spatial precision
Nature Communications (2020)