Abstract
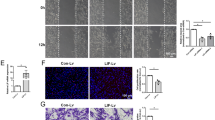
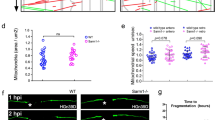
Axons in the adult mammalian central nervous system (CNS) exhibit little regeneration after injury. It has been suggested that several axonal growth inhibitors prevent CNS axonal regeneration. Recent research has demonstrated that semaphorin3A (Sema3A) is one of the major inhibitors of axonal regeneration. We identified a strong and selective inhibitor of Sema3A, SM-216289, from the fermentation broth of a fungal strain. To examine the effect of SM-216289 in vivo, we transected the spinal cord of adult rats and administered SM-216289 into the lesion site for 4 weeks. Rats treated with SM-216289 showed substantially enhanced regeneration and/or preservation of injured axons, robust Schwann cell–mediated myelination and axonal regeneration in the lesion site, appreciable decreases in apoptotic cell number and marked enhancement of angiogenesis, resulting in considerably better functional recovery. Thus, Sema3A is essential for the inhibition of axonal regeneration and other regenerative responses after spinal cord injury (SCI). These results support the possibility of using Sema3A inhibitors in the treatment of human SCI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schwab, M.E., Kapfhammer, J.P. & Bandtlow, C.E. Inhibitors of neurite growth. Annu. Rev. Neurosci. 16, 565–595 (1993).
GrandPre, T., Nakamura, F., Vartanian, T. & Strittmatter, S.M. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature 403, 439–444 (2000).
Chen, M.S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
Domeniconi, M. et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35, 283–290 (2002).
Olson, L. Medicine: clearing a path for nerve growth. Nature 416, 589–590 (2002).
Wang, K.C. et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002).
Li, S. et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 24, 10511–10520 (2004).
Kim, J.E., Li, S., GrandPre, T., Qiu, D. & Strittmatter, S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38, 187–199 (2003).
Simonen, M. et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38, 201–211 (2003).
Zheng, B. et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38, 213–224 (2003).
Kim, J.E., Liu, B.P., Park, J.H. & Strittmatter, S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 44, 439–451 (2004).
Zheng, B. et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl. Acad. Sci. USA 102, 1205–1210 (2005).
Pasterkamp, R.J. et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell. Neurosci. 13, 143–166 (1999).
Pasterkamp, R.J., Anderson, P.N. & Verhaagen, J. Peripheral nerve injury fails to induce growth of lesioned ascending column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 13, 457–471 (2001).
Bradbury, E.J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
De Winter, F. et al. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 175, 61–75 (2002).
Morgenstern, D.A., Asher, R.A. & Fawcett, J.W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 137, 313–332 (2002).
Silver, J. & Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004).
Kolodkin, A.L., Matthes, D.J. & Goodman, C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399 (1993).
Luo, Y., Raible, D. & Raper, J.A. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75, 217–227 (1993).
Goshima, Y., Nakamura, F., Strittmatter, P. & Strittmatter, S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to unc-33. Nature 376, 509–514 (1995).
Taniguchi, M. et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19, 519–530 (1997).
Sasaki, Y. et al. Fyn and Cdk5 mediate semaphorin-3A signaling which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35, 907–920 (2002).
Uchida, Y. et al. Semaphorin-3A signaling is mediated via sequential Cdk5 and GSK3b phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes Cells 10, 165–179 (2005).
Kikuchi, K. et al. In vitro and in vivo characterization of a novel Semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J. Biol. Chem. 278, 42985–42991 (2003).
Kumagai, K., Hosotani, N., Kikuchi, K., Kimura, T. & Saji, I. Xanthofulvin, a novel semaphorin inhibitor produced by a strain of Penicillium. J. Antibiot. 56, 610–616 (2003).
He, Z. & Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell 90, 739–751 (1997).
Kitsukawa, T. et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19, 995–1005 (1997).
Kolodkin, A.L. et al. Neuropilin is a Semaphorin III receptor. Cell 90, 753–762 (1997).
Takahashi, T. et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69 (1999).
Tamagnone, L. et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80 (1999).
Yaron, A., Huang, P.H., Cheng, H.J. & Tessier-Lavigne, M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 45, 513–523 (2005).
Christensen, M.D. & Hulsebosch, C.E. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 147, 463–475 (1997).
Romero, M.I. et al. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 20, 4435–4445 (2000).
Romero, M.I., Rangappa, N., Garry, M.G. & Smith, G.M. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J. Neurosci. 21, 8408–8416 (2001).
Bregman, B.S. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 431, 265–279 (1987).
Eickholt, B.J., Mackenzie, S.L., Graham, A., Walsh, F.S. & Doherty, P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development 126, 2181–2189 (1999).
Bagnard, D. et al. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J. Neurosci. 21, 3332–3341 (2001).
Bagri, A. & Tessier-Lavigne, M. Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Adv. Exp. Med. Biol. 515, 13–31 (2002).
Archelos, J.J. et al. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J. Neurosci. Res. 35, 46–53 (1993).
Basso, D.M., Beattie, M.S. & Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 (1995).
Mikami, Y. et al. A simple and reliable behavioral analysis of locomotor function after spinal cord injury in mice. Technical note. J. Neurosurg. 97, 142–147 (2002).
Bareyre, F.M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 (2004).
Morrison, S.J., White, P.M., Zock, C. & Anderson, D.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96, 737–749 (1999).
Cai, D., Shen, Y., De Bellard, M., Tang, S. & Filbin, M.T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22, 89–101 (1999).
McTigue, D.M., Horner, P.J., Stokes, B.T. & Gage, F.H. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J. Neurosci. 18, 5354–5365 (1998).
Ikegami, T. et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur. J. Neurosci. 22, 3036–3046 (2005).
Okada, S. et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834 (2006).
Guthrie, S. & Lumsden, A. Collagen gel coculture of neural tissue. Neuroprotocols 4, 116–120 (1994).
Thomaidou, D. et al. Soluble forms of NCAM and F3 neuronal cell adhesion molecules promote Schwann cell migration: identification of protein tyrosine phosphatases zeta/beta as the putative F3 receptors on Schwann cells. J. Neurochem. 78, 767–778 (2001).
Acknowledgements
We are grateful to L. Benowitz (Children's Hospital Boston); H. Fujisawa (Nagoya University); A.L. Kolodkin (Johns Hopkins University); and Y. Ihara and K. Mori (Tokyo University) for reagents. We also thank M. Dezawa, S. Kawabata, U. Uchida, Y. Sugiyama, F. Nakamura, T. Nagai, S. Miyao, T. Harada, K. Watanabe, H. Hanafusa, Y. Ujimasa, T. Yagi and G. Yiu for technical assistance. We are also grateful to T. Takahashi, H. Fujisawa and F. Murakami for their critical reading of the manuscript, to the members of the Okano Laboratory for their comments on the manuscript. This work was supported by grants from the Leading Project for Realization of Regenerative Medicine from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; the Japan Science and Technology Corporation (JST); and the General Insurance Association of Japan. This work was also supported by a Keio University special grant-in-aid for innovative collaborative research projects to H.O.; a Keio University grant-in-aid for encouragement of young medical scientists to S.K. and A.I.; and a grant-in-aid from the 21st Century COE Program of MEXT, Japan, to Keio University.
Author information
Authors and Affiliations
Contributions
S.K. wrote the manuscript, conducted all the SCI experiments (including preparation of the SCI model rats, and behavioral and histochemical characterizations), conducted in vitro assays (including growth cone collapse assay) and all the immunoblotting, and prepared the recombinant proteins. A.I. conducted rat SCI experiments as detailed above, conducted SCI experiments of Sema3a-deficient mice, and cowrote the manuscript. M.N. instructed S.K. and A.I. on the technical aspects of the SCI experiments, and cowrote the manuscript. A.K. conducted histochemical characterization of SCI model rats. K. Kikuchi conducted in vitro experiments (including collagen gel coculture, KB-cell cell growth assay and Schwann cell migration assay). S.S. conducted cell preparation for the in vitro migration assay. H.J.O. prepared the recombinant proteins. T.I. participated in SCI experiments including behavioral characterizations. A.M. prepared the recombinant semaphorin proteins. O.K participated in histochemical characterization of SCI model rats. C.N. organized the collaboration with Keio University at Dainippon Sumitomo. K. Kumagai prepared SM-216289 and examined its specificity of action in vitro. T.K. supervised the SM-216289 project at Dainippon Sumitomo. Y.S. and Y.G. examined the specificity of SM-216289's effect on Sema3A by growth cone collapse assay. M.T. provided and conducted the genetic diagnosis of Sema3a-deficient mice. M.I. conducted the in vitro fertilization of Sema3a-deficient mice. Z.H. provided advice on the experimental design. Y.T. supported and supervised the SCI experiments conducted at Keio University. H.O. supervised the whole project and cowrote the manuscript with S.K., A.I. and M.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression of NP-1 after SCI. (PDF 1266 kb)
Supplementary Fig. 2
SM-216289 strongly inhibits Sema3A in vivo in the injured spinal cord. (PDF 214 kb)
Supplementary Fig. 3
Effect of SM-216289 upon sensory neurons in the lesion site after SCI. (PDF 541 kb)
Supplementary Fig. 4
Expression of Sema3A receptor components in fibloblast cell line, L-929 cells. . (PDF 995 kb)
Supplementary Fig. 5
SM-216289 reduces apoptosis and enhances angiogenesis in the injured spinal cord. (PDF 632 kb)
Supplementary Fig. 6
SM-216289-induced functional recovery after SCI was attenuated by retransection or administration of 5, 7-DHT. (PDF 403 kb)
Supplementary Table 1
Pharmacological profile of SM-216289. Summary of IC50 values for receptor and ion channel binding assays, and enzyme and kinase inhibition tests. (PDF 1347 kb)
Rights and permissions
About this article
Cite this article
Kaneko, S., Iwanami, A., Nakamura, M. et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 12, 1380–1389 (2006). https://doi.org/10.1038/nm1505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1505
This article is cited by
-
Mertk Reduces Blood-Spinal Cord Barrier Permeability Through the Rhoa/Rock1/P-MLC Pathway After Spinal Cord Injury
Neuroscience Bulletin (2024)
-
Astrocytic Extracellular Vesicles Regulated by Microglial Inflammatory Responses Improve Stroke Recovery
Molecular Neurobiology (2024)
-
Hepatocyte growth factor pretreatment boosts functional recovery after spinal cord injury through human iPSC-derived neural stem/progenitor cell transplantation
Inflammation and Regeneration (2023)
-
Axon guidance gene-targeted siRNA delivery system improves neural stem cell transplantation therapy after spinal cord injury
Biomaterials Research (2023)
-
Regulation of axonal regeneration after mammalian spinal cord injury
Nature Reviews Molecular Cell Biology (2023)