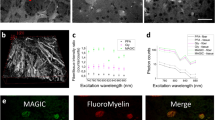
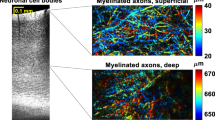
Abstract
We report a newly developed technique for high-resolution in vivo imaging of myelinated axons in the brain, spinal cord and peripheral nerve that requires no fluorescent labeling. This method, based on spectral confocal reflectance microscopy (SCoRe), uses a conventional laser-scanning confocal system to generate images by merging the simultaneously reflected signals from multiple lasers of different wavelengths. Striking color patterns unique to individual myelinated fibers are generated that facilitate their tracing in dense axonal areas. These patterns highlight nodes of Ranvier and Schmidt-Lanterman incisures and can be used to detect various myelin pathologies. Using SCoRe we carried out chronic brain imaging up to 400 μm deep, capturing de novo myelination of mouse cortical axons in vivo. We also established the feasibility of imaging myelinated axons in the human cerebral cortex. SCoRe adds a powerful component to the evolving toolbox for imaging myelination in living animals and potentially in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nave, K.-A. Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010).
Zatorre, R.J., Fields, R.D. & Johansen-Berg, H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536 (2012).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Franklin, R.J.M. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Fancy, S.P.J., Chan, J.R., Baranzini, S.E., Franklin, R.J.M. & Rowitch, D.H. Myelin regeneration: a recapitulation of development? Annu. Rev. Neurosci. 34, 21–43 (2011).
Bartzokis, G. et al. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol. Psychiatry 72, 1026–1034 (2012).
Kirby, B.B. et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9, 1506–1511 (2006).
Kaya, F. et al. Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair. J. Neurosci. 32, 12885–12895 (2012).
Wang, H., Fu, Y., Zickmund, P., Shi, R. & Cheng, J.-X. Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys. J. 89, 581–591 (2005).
Fu, Y., Huff, T.B., Wang, H.-W., Wang, H. & Cheng, J.-X. Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy. Opt. Express 16, 19396–19409 (2008).
Imitola, J. et al. Multimodal coherent anti-Stokes Raman scattering microscopy reveals microglia-associated myelin and axonal dysfunction in multiple sclerosis-like lesions in mice. J. Biomed. Opt. 16, 021109 (2011).
Ben Arous, J. et al. Single myelin fiber imaging in living rodents without labeling by deep optical coherence microscopy. J. Biomed. Opt. 16, 116012 (2011).
Witte, S. et al. Label-free live brain imaging and targeted patching with third-harmonic generation microscopy. Proc. Natl. Acad. Sci. USA 108, 5970–5975 (2011).
Farrar, M.J., Wise, F.W., Fetcho, J.R. & Schaffer, C.B. In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy. Biophys. J. 100, 1362–1371 (2011).
Filler, T.J. & Peuker, E.T. Reflection contrast microscopy (RCM): a forgotten technique? J. Pathol. 190, 635–638 (2000).
Xiao, J., Levitt, J.B. & Buffenstein, R. The use of a novel and simple method of revealing neural fibers to show the regression of the lateral geniculate nucleus in the naked mole-rat (Heterocephalus glaber). Brain Res. 1077, 81–89 (2006).
Grutzendler, J., Kasthuri, N. & Gan, W.-B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Waxman, S.G. Ultrastructural observations on branching patterns of central axons. Neurosci. Lett. 1, 251–256 (1975).
Ha, H. Axonal bifurcation in the dorsal root ganglion of the cat: a light and electron microscopic study. J. Comp. Neurol. 140, 227–240 (1970).
Hirrlinger, P.G. et al. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol. Cell. Neurosci. 30, 291–303 (2005).
Jacobson, S. Sequence of myelination in the brain of the albino rat. A. Cerebral cortex, thalamus and related structures. J. Comp. Neurol. 121, 5–29 (1963).
Chong, S.Y.C. et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl. Acad. Sci. USA 109, 1299–1304 (2012).
Binding, J. et al. Brain refractive index measured in vivo with high-NA defocus-corrected full-field OCT and consequences for two-photon microscopy. Opt. Express 19, 4833–4847 (2011).
Macleod, H.A. Thin-Film Optical Filters (Institute of Physics Publishing, 2001).
Balice-Gordon, R.J., Bone, L.J. & Scherer, S.S. Functional gap junctions in the schwann cell myelin sheath. J. Cell Biol. 142, 1095–1104 (1998).
Gould, R.M., Byrd, A.L. & Barbarese, E. The number of Schmidt-Lanterman incisures is more than doubled in shiverer PNS myelin sheaths. J. Neurocytol. 24, 85–98 (1995).
Lam, C.K., Yoo, T., Hiner, B., Liu, Z. & Grutzendler, J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465, 478–482 (2010).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Harb, R., Whiteus, C., Freitas, C. & Grutzendler, J. In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 33, 146–156 (2013).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Gan, W.B., Grutzendler, J., Wong, W.T., Wong, R.O. & Lichtman, J.W. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27, 219–225 (2000).
Hong, G. et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 18, 1841–1846 (2012).
Gupta, N. et al. Neural stem cell engraftment and myelination in the human brain. Sci. Transl. Med. 4, 155ra137 (2012).
Windrem, M.S. et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565 (2008).
Bø, L., Vedeler, C.A., Nyland, H.I., Trapp, B.D. & Mørk, S.J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 62, 723–732 (2003).
Lucchinetti, C.F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Nimmerjahn, A., Kirchhoff, F., Kerr, J.N.D. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1, 31–37 (2004).
Fenrich, K.K. et al. Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J. Physiol. (Lond.) 590, 3665–3675 (2012).
Acknowledgements
This study was supported by the following grants: R01AG027855 and R01HL106815 (J.G.). We would like to thank J. Bewersdorf and D. Toomre for helpful discussions and P. Yuan for critical reading of the manuscript. We thank A. Nishiyama (University of Connecticut) and F. Kirchhoff (University of Saarland) for providing PLPDsRed mice. Postmortem human specimen was obtained from the brain bank in the Cognitive Neurology and Alzheimer's disease Center (CNADC) at Northwestern University (grant AG13854).
Author information
Authors and Affiliations
Contributions
A.J.S. and J.G. conceived and designed the initial project. A.J.S. and R.A.H. carried out the experiments. All authors contributed to experimental design, data analysis and manuscript. J.G. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 15505 kb)
Three dimensional rendering of a SCoRe image captured in vivo in a Thy1-YFP transgenic mouse.
A single YFP-labeled (green) reflective (magenta) axon (arrow) is shown among many non myelinated YFP-labeled axons and dendrites. (AVI 8154 kb)
Representative 450 μm z stack of the mouse cortex acquired in vivo with SCoRe.
Video demonstrates the depth capabilities of SCoRe which maintains a high signal to noise ratio even at ~400 μm. The decrease in the number of reflected fibers with depth indicates the drop-off in the number of myelinated fibers just below cortical layer 1 projection axons in addition to the change in the orientation of some of the myelinated axons as SCoRe is not able to efficiently detect myelinated axons running orthogonally. Step size 1 μm, depth indicated in upper left corner and video displayed at 10 frames per second. (AVI 23477 kb)
Three dimensional rendering of a SCoRe image from the sciatic nerve.
Video shows the unique reflected spectrum from individual axons after removing the layers of the highly reflective and disordered signal from the sciatic epineurium. (AVI 6094 kb)
Rights and permissions
About this article
Cite this article
Schain, A., Hill, R. & Grutzendler, J. Label-free in vivo imaging of myelinated axons in health and disease with spectral confocal reflectance microscopy. Nat Med 20, 443–449 (2014). https://doi.org/10.1038/nm.3495
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3495
This article is cited by
-
Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice
Nature Neuroscience (2023)
-
Norepinephrine modulates calcium dynamics in cortical oligodendrocyte precursor cells promoting proliferation during arousal in mice
Nature Neuroscience (2023)
-
Computational conjugate adaptive optics microscopy for longitudinal through-skull imaging of cortical myelin
Nature Communications (2023)
-
Contribution of Intravital Neuroimaging to Study Animal Models of Multiple Sclerosis
Neurotherapeutics (2023)
-
Cortical neurons exhibit diverse myelination patterns that scale between mouse brain regions and regenerate after demyelination
Nature Communications (2021)