Abstract
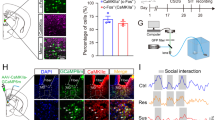
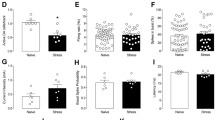
The hippocampus and prefrontal cortex (PFC) are connected in a reciprocal manner: whereas the hippocampus projects directly to the PFC, a polysynaptic pathway that passes through the nucleus reuniens (RE) of the thalamus relays inputs from the PFC to the hippocampus. The present study demonstrates that lesioning and/or inactivation of the RE reduces coherence in the PFC–hippocampal pathway, provokes an antidepressant-like behavioral response in the forced swim test and prevents, but does not ameliorate, anhedonia in the chronic mild stress (CMS) model of depression. Additionally, RE lesioning before CMS abrogates the well-known neuromorphological and endocrine correlates of CMS. In summary, this work highlights the importance of the reciprocal connectivity between the hippocampus and PFC in the establishment of stress-induced brain pathology and suggests a role for the RE in promoting resilience to depressive illness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Duman RS, Aghajanian GK . Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338: 68–72.
Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N . The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 2007; 27: 2781–2787.
Price JL, Drevets WC . Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 2012; 16: 61–71.
Spinelli S, Muller T, Friedel M, Sigrist H, Lesch KP, Henkelman M et al. Effects of repeated adolescent stress and serotonin transporter gene partial knockout in mice on behaviors and brain structures relevant to major depression. Front Behav Neurosci 2013; 7: 215.
Oliveira JF, Dias NS, Correia M, Gama-Pereira F, Sardinha VM, Lima A et al. Chronic stress disrupts neural coherence between cortico-limbic structures. Front Neural Circuits 2013; 7: 10.
Siapas AG, Lubenov EV, Wilson MA . Prefrontal phase locking to hippocampal theta oscillations. Neuron 2005; 46: 141–151.
Vertes RP . Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol 2002; 442: 163–187.
Xu W, Sudhof TC . A neural circuit for memory specificity and generalization. Science 2013; 339: 1290–1295.
Di Prisco GV, Vertes RP . Excitatory actions of the ventral midline thalamus (rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse 2006; 60: 45–55.
Zhang Y, Yoshida T, Katz DB, Lisman JE . NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J Neurophysiol 2012; 107: 3181–3189.
Zimmerman EC, Grace AA . The nucleus reuniens of the midline thalamus gates prefrontal-hippocampal modulation of ventral tegmental area dopamine neuron activity. J Neurosci 2016; 36: 8977–8984.
Layfield DM, Patel M, Hallock H, Griffin AL . Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiol Learn Mem 2015; 125: 163–167.
Hallock HL, Wang A, Griffin AL . Ventral midline thalamus is critical for hippocampal-prefrontal synchrony and spatial working memory. J Neurosci 2016; 36: 8372–8389.
Davoodi FG, Motamedi F, Akbari E, Ghanbarian E, Jila B . Effect of reversible inactivation of reuniens nucleus on memory processing in passive avoidance task. Behav Brain Res 2011; 221: 1–6.
Polissidis A, Chouliara O, Galanopoulos A, Rentesi G, Dosi M, Hyphantis T et al. Individual differences in the effects of cannabinoids on motor activity, dopaminergic activity and DARPP-32 phosphorylation in distinct regions of the brain. Int J Neuropsychopharmacol 2009; 13: 1175–1191.
Hembrook JR, Mair RG . Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus 2011; 21: 815–826.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates5th edn. Elsevier Academic Press: Amsterdam, The Netherlaands; Boston, MA, USA, 2005.
Dolleman-van der Weel MJ, Morris RG, Witter MP . Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct 2009; 213: 329–342.
Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B et al. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci 2012; 32: 9947–9959.
Prasad JA, Macgregor EM, Chudasama Y . Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct 2013; 218: 85–96.
Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D et al. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci 2013; 33: 8772–8783.
Rocher C, Spedding M, Munoz C, Jay TM . Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex 2004; 14: 224–229.
Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S et al. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav 2008; 93: 595–605.
Kokras N, Antoniou K, Dalla C, Bekris S, Xagoraris M, Ovestreet DH et al. Sex-related differential response to clomipramine treatment in a rat model of depression. J Psychopharmacol 2009; 23: 945–956.
Kokras N, Dalla C, Sideris AC, Dendi A, Mikail HG, Antoniou K et al. Behavioral sexual dimorphism in models of anxiety and depression due to changes in HPA axis activity. Neuropharmacology 2012; 62: 436–445.
Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience 2004; 126: 849–857.
Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C . Forced swim test: what about females? Neuropharmacology 2015; 99: 408–421.
Cryan JF, Markou A, Lucki I . Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 2002; 23: 238–245.
Mikail HG, Dalla C, Kokras N, Kafetzopoulos V, Papadopoulou-Daifoti Z . Sertraline behavioral response associates closer and dose-dependently with cortical rather than hippocampal serotonergic activity in the rat forced swim stress. Physiol Behav 2012; 107: 201–206.
Detke MJ, Rickels M, Lucki I . Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995; 121: 66–72.
Ventura-Silva AP, Pego JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F et al. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci 2012; 36: 3396–3406.
Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A et al. Chronic mild stress impact: are females more vulnerable? Neuroscience 2005; 135: 703–714.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 2009; 14: 764–773, 739.
Pitychoutis PM, Dalla C, Sideris AC, Tsonis PA, Papadopoulou-Daifoti Z . 5-HT(1A), 5-HT(2A), and 5-HT(2C) receptor mRNA modulation by antidepressant treatment in the chronic mild stress model of depression: sex differences exposed. Neuroscience 2012; 210: 152–167.
Willner P . Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005; 52: 90–110.
Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z . Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res 2005; 161: 45–59.
Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience 2008; 152: 656–669.
Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N . Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex 2007; 17: 1998–2006.
Dalla C, Whetstone AS, Hodes GE, Shors TJ . Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett 2009; 449: 52–56.
Harris KM . Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol 1999; 9: 343–348.
Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S . Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 2012; 37: 2210–2221.
Varela F, Lachaux JP, Rodriguez E, Martinerie J . The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2001; 2: 229–239.
Cryan JF, Page ME, Lucki I . Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005; 182: 335–344.
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 2011; 16: 1177–1188.
Khemissi W, Farooq RK, Le Guisquet AM, Sakly M, Belzung C . Dysregulation of the hypothalamus-pituitary-adrenal axis predicts some aspects of the behavioral response to chronic fluoxetine: association with hippocampal cell proliferation. Front Behav Neurosci 2014; 8: 340.
Belzung C, Billette de Villemeur E . The design of new antidepressants: can formal models help? A first attempt using a model of the hippocampal control over the HPA-axis based on a review from the literature. Behav Pharmacol 2010; 21: 677–689.
Holsboer-Trachsler E, Stohler R, Hatzinger M . Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res 1991; 38: 163–171.
Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C . Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull 2007; 71: 601–609.
Connor TJ, Kelly JP, Leonard BE . Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol Biochem Behav 1997; 58: 961–967.
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ . Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 1995; 64: 477–505.
Slattery DA, Neumann ID, Cryan JF . Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. J Psychopharmacol 2011; 25: 1295–1303.
Kokras N, Dalla C . Sex differences in animal models of psychiatric disorders. Br J Pharmacol 2014; 171: 4595–4619.
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 2009; 325: 621–625.
Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ . Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 2006; 499: 768–796.
Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF . Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA 2009; 106: 17957–17962.
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874.
Perez-Cruz C, Muller-Keuker JI, Heilbronner U, Fuchs E, Flugge G . Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plast 2007; 2007: 46276.
Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biol Psychiatry 2007; 62: 47–54.
Drexel M, Preidt AP, Kirchmair E, Sperk G . Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience 2011; 189: 316–329.
Lara-Vasquez A, Espinosa N, Duran E, Stockle M, Fuentealba P . Midline thalamic neurons are differentially engaged during hippocampus network oscillations. Sci Rep 2016; 6: 29807.
Acknowledgements
We thank the excellent technical assistance provided by Mrs D Papassava and Mrs Goreti Pinto. We also thank Dr R Matsas and Dr M Thomaidou (Athens Pasteur Institute), Dr S Pagakis (BRFAA) for providing equipment and Specifar S.A., Greece for providing sertraline. This work was supported by an ‘Education and Lifelong Learning, Supporting Postdoctoral Researchers’, co-financed by the European Social Fund (ESF) and the General Secretariat for Research and Technology, Greece, the Life and Health Sciences Research Institute (ICVS), ON.2—O NOVO NORTE—North Portugal Regional Operational Program 2007/2013 of the National Strategic Reference Framework (NSRF) 2007/2013 through the European Regional Development Fund (ERDF), the Portuguese Foundation for Science and Technology (FCT; grant no. NMC-113934) and an InEurope program funded by International Brain Research Organization. NK was funded for the FST behavioral scoring software by an IKY-Siemens Fellowship of Excellence for Postgraduate Studies. VMS and JFO were funded by FCT and Marie Curie IEF fellowships as well as the Bial Foundation.
Author contributions
VK contributed to the design of the study, performed all experimental procedures, statistical analyses and compiled the first draft. NK contributed to the design of the study, the analysis and interpretation of results and, with AV, participated in some of the experiments; JFO and VMS helped with the electrophysiological analyses. IS and HL-A contributed to the histochemical analyses. IS and OFXA helped with the studies involving stress and data interpretation. ZP-D, KA and NS participated in study design and interpretation of results and provided significant insights. CD supervised and contributed to all parts of this project. All authors contributed to the writing of the manuscript and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Kafetzopoulos, V., Kokras, N., Sotiropoulos, I. et al. The nucleus reuniens: a key node in the neurocircuitry of stress and depression. Mol Psychiatry 23, 579–586 (2018). https://doi.org/10.1038/mp.2017.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.55
This article is cited by
-
Somatostatin-Positive Neurons in the Rostral Zona Incerta Modulate Innate Fear-Induced Defensive Response in Mice
Neuroscience Bulletin (2023)
-
Nucleus reuniens inactivation reverses stress-induced hypodopaminergic state and altered hippocampal-accumbens synaptic plasticity
Neuropsychopharmacology (2022)
-
Chronic mild stress paradigm as a rat model of depression: facts, artifacts, and future perspectives
Psychopharmacology (2022)
-
The role of the nucleus reuniens in regulating contextual conditioning with the predator odor TMT in female rats
Psychopharmacology (2021)
-
Hippocampal cytogenesis abrogation impairs inter-regional communication between the hippocampus and prefrontal cortex and promotes the time-dependent manifestation of emotional and cognitive deficits
Molecular Psychiatry (2021)