Abstract
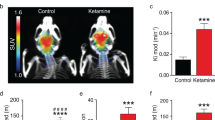
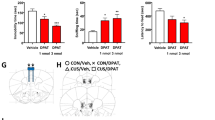
Several drugs have recently been reported to induce rapid antidepressant effects in clinical trials and rodent models. Although the cellular mechanisms involved remain unclear, reports suggest that increased glutamate transmission contributes to these effects. Here, we demonstrate that the antidepressant-like efficacy of three unique drugs, with reported rapid onset antidepressant properties, is coupled with a rapid transient rise in glutamate cycling in the medial prefronal cortex (mPFC) of awake rats as measured by ex vivo 1H-[13C]-nuclear magnetic resonance spectroscopy. Rats were acutely pretreated by intraperitoneal injection with a single dose of ketamine (1, 3, 10, 30 and 80 mg kg−1), Ro 25-6981 (1, 3 and 10 mg kg−1), scopolamine (5, 25 and 100 μg kg−1) or vehicle (controls). At fixed times after drug injection, animals received an intravenous infusion of [1,6-13C2]glucose for 8 min to enrich the amino-acid pools of the brain with 13C, followed by rapid euthanasia. The mPFC was dissected, extracted with ethanol and metabolite 13C enrichments were measured. We found a clear dose-dependent effect of ketamine and Ro 25-6981 on behavior and the percentage of 13C enrichment of glutamate, glutamine and GABA (γ-aminobutyric acid). Further, we also found an effect of scopolamine on both cycling and behavior. These studies demonstrate that three pharmacologically distinct classes of drugs, clinically related through their reported rapid antidepressant actions, share the common ability to rapidly stimulate glutamate cycling at doses pertinent for their antidepressant-like efficacy. We conclude that increased cycling precedes the antidepressant action at behaviorally effective doses and suggest that the rapid change in cycling could be used to predict efficacy of novel agents or identify doses with antidepressant activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Martinowich K, Jimenez DV, Zarate CA Jr, Manji HK . Rapid antidepressant effects: moving right along. Mol Psychiatry 2013; 18: 856–863.
Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 2015; 172: 950–966.
Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry 2014; 9: 978–985.
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW . An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 2008; 28: 631–637.
Drevets WC, Zarate CA Jr, Furey ML . Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol psychiatry 2013; 73: 1156–1163.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95.
Koike H, Iijima M, Chaki S . Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 2011; 224: 107–111.
Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013; 74: 742–749.
Moghaddam B, Adams B, Verma A, Daly D . Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927.
Homayoun H, Moghaddam B . NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 2007; 27: 11496–11500.
Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G . (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry 2012; 71: 1022–1025.
Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther 2014; 351: 448–456.
Chowdhury GM, Patel AB, Mason GF, Rothman DL, Behar KL . Glutamatergic and GABAergic neurotransmitter cycling and energy metabolism in rat cerebral cortex during postnatal development. J Cereb Blood Flow Metab 2007; 27: 1895–1907.
Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG . Neuronal–glial glucose oxidation and glutamatergic–GABAergic function. J Cereb Blood Flow Metab 2006; 26: 865–877.
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D . Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 1997; 154: 805–811.
Nishizawa N, Nakao S, Nagata A, Hirose T, Masuzawa M, Shingu K . The effect of ketamine isomers on both mice behavioral responses and c-Fos expression in the posterior cingulate and retrosplenial cortices. Brain Res 2000; 857: 188–192.
Littlewood CL, Jones N, O'Neill MJ, Mitchell SN, Tricklebank M, Williams SC . Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology 2006; 186: 64–81.
Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology 2008; 197: 401–408.
Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE . Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology 2000; 22: 400–412.
Miyamoto S, Mailman RB, Lieberman JA, Duncan GE . Blunted brain metabolic response to ketamine in mice lacking D(1 A) dopamine receptors. Brain Res 2001; 894: 167–180.
Duncan GE, Moy SS, Knapp DJ, Mueller RA, Breese GR . Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Res 1998; 787: 181–190.
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 2008; 63: 349–352.
Haller J, Nagy R, Toth M, Pelczer KG, Mikics E . NR2B subunit-specific NMDA antagonist Ro25-6981 inhibits the expression of conditioned fear: a comparison with the NMDA antagonist MK-801 and fluoxetine. Behav Pharmacol 2011; 22: 113–121.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-d-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Shaffer CL, Osgood SM, Smith DL, Liu J, Trapa PE . Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology 2014; 86: 174–180.
Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Domino SE . Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesth Analg 1982; 61: 87–92.
Lucki I . The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 1997; 8: 523–532.
Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J et al. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR Biomed 2009; 22: 737–744.
Gao XM, Shirakawa O, Du F, Tamminga CA . Delayed regional metabolic actions of phencyclidine. Eur J Pharmacol 1993; 241: 7–15.
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 2005; 162: 394–396.
Homayoun H, Jackson ME, Moghaddam B . Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol 2005; 93: 1989–2001.
Nagy D, Stoiljkovic M, Menniti FS, Hajos M . Differential effects of an NR2B NAM and ketamine on synaptic potentiation and gamma synchrony: relevance to rapid-onset antidepressant efficacy. Neuropsychopharmacology 2015; e-pub ahead of print 21 October 2015.
Duman RS, Aghajanian GK . Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338: 68–72.
Acknowledgements
We thank Amy Newton and Yulia Benitex of Bristol-Myers Squibb for the execution of the rat ketamine PK studies, and Terry Nixon, Peter Brown and Scott McIntyre for their support in maintaining the NMR spectrometer. This work was supported by National Institute of Mental Health R01-MH095104 and R01-MH081211, NARSAD, QNMR Core Center P30-NS052519, The VA National Center for PTSD and funding from Bristol-Myers Squib.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Dr Sanacora has received consulting fees from AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly & Co., Hoffman La-Roche, Merck, Navigen, Naurex, Noven Pharmaceuticals, Servier Pharmaceuticals, Takeda, Teva and Vistagen therapeutics over the past 24 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex and Servier over the past 24 months. Free medication was provided to Dr Sanacora for an NIH-sponsored study by Sanofi-Aventis. In addition, he holds shares in BioHaven Pharmaceuticals Holding Company and is a coinventor on a US patent (no. 8 778 979) held by the Yale University. Dr Duman has received consulting fees from Taisho, Naurex, Sunovion and Johnson & Johnson, and investigator-initiated grants from Forest, Naurex, Sunovion and Eli Lilly & Co. Dr Bristow is an employee of Bristol-Myers Squibb. Dr Schaeffer was an employee of Bristol-Myers Squibb at the time the research was completed and is currently an employee of Janssen Research and Development. Dr Banasr has received research contracts from BioHaven Pharmaceuticals and Servier Pharmaceuticals. Dr Behar holds common stock in Pfizer. The remaining authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Chowdhury, G., Zhang, J., Thomas, M. et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22, 120–126 (2017). https://doi.org/10.1038/mp.2016.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.34
This article is cited by
-
M1 acetylcholine receptors in somatostatin interneurons contribute to GABAergic and glutamatergic plasticity in the mPFC and antidepressant-like responses
Neuropsychopharmacology (2023)
-
Region- and time- specific effects of ketamine on cerebral blood flow: a randomized controlled trial
Neuropsychopharmacology (2023)
-
Ketamine and Zinc: Treatment of Anorexia Nervosa Via Dual NMDA Receptor Modulation
CNS Drugs (2023)
-
Imaging the effect of ketamine on synaptic density (SV2A) in the living brain
Molecular Psychiatry (2022)
-
Novel approaches to estimate prefrontal synaptic strength in vivo in humans: of relevance to depression, schizophrenia, and ketamine
Neuropsychopharmacology (2022)