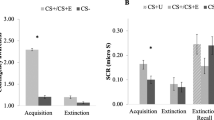
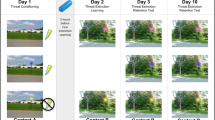
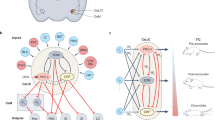
Abstract
Overgeneralization of conditioned threat responses is a robust clinical marker of anxiety disorders. In overgeneralization, responses that are appropriate to threat-predicting cues are evoked by perceptually similar safety-predicting cues. Inappropriate learning of conditioned threat responses may thus form an etiological basis for anxiety disorders. The role of dopamine (DA) in memory encoding is well established. Indeed by signaling salience and valence, DA is thought to facilitate discriminative learning between stimuli representing safety or threat. However, the neuroanatomical and biochemical substrates through which DA modulates overgeneralization of threat responses remain poorly understood. Here we report that the modulation of DA D2 receptor (D2R) signaling bidirectionally regulates the consolidation of fear responses. While the blockade of D2R induces generalized threat responses, its stimulation facilitates discriminative learning between stimuli representing safety or threat. Moreover, we show that controlled threat generalization requires the coordinated activation of D2R in the bed nucleus of the stria terminalis and the central amygdala. Finally, we identify the mTORC1 cascade activation as an important molecular event by which D2R mediates its effects. These data reveal that D2R signaling in the extended amygdala constitutes an important checkpoint through which DA participates in the control of threat processing and the emergence of overgeneralized threat responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fox AS, Oler JA, Tromp do PM, Fudge JL, Kalin NH . Extending the amygdala in theories of threat processing. Trends Neurosci 2015; 38: 319–329.
Davis M, Walker DL, Miles L, Grillon C . Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 2010; 35: 105–135.
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010; 468: 277–282.
Duvarci S, Bauer EP, Pare D . The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci 2009; 29: 10357–10361.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010; 468: 270–276.
Dunsmoor JE, Paz R . Fear generalization and anxiety: behavioral and neural mechanisms. Biol Psychiatry 2015; 78: 336–343.
Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther 2005; 43: 1391–1424.
Ghosh S, Chattarji S . Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci 2015; 18: 112–120.
Hasue RH, Shammah-Lagnado SJ . Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol 2002; 454: 15–33.
Bromberg-Martin ES, Matsumoto M, Hikosaka O . Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010; 68: 815–834.
Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H . Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci 2008; 28: 11673–11684.
Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci 2011; 14: 620–626.
Fadok JP, Dickerson TM, Palmiter RD . Dopamine is necessary for cue-dependent fear conditioning. J Neurosci 2009; 29: 11089–11097.
Greba Q, Gifkins A, Kokkinidis L . Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res 2001; 899: 218–226.
Guarraci FA, Frohardt RJ, Falls WA, Kapp BS . The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behav Neurosci 2000; 114: 647–651.
Guarraci FA, Frohardt RJ, Young SL, Kapp BS . A functional role for dopamine transmission in the amygdala during conditioned fear. Ann N Y Acad Sci 1999; 877: 732–736.
Gonzalez MC, Kramar CP, Tomaiuolo M, Katche C, Weisstaub N, Cammarota M et al. Medial prefrontal cortex dopamine controls the persistent storage of aversive memories. Front Behav Neurosci 2014; 8: 408.
Gozzani JL, Izquierdo I . Possible peripheral adrenergic and central dopaminergic influences in memory consolidation. Psychopharmacology (Berl) 1976; 49: 109–111.
Lalumiere RT, Nguyen LT, McGaugh JL . Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. Eur J Neurosci 2004; 20: 2804–2810.
Manago F, Castellano C, Oliverio A, Mele A, De Leonibus E . Role of dopamine receptors subtypes, D1-like and D2-like, within the nucleus accumbens subregions, core and shell, on memory consolidation in the one-trial inhibitory avoidance task. Learn Mem 2009; 16: 46–52.
Maroun M, Akirav I . Differential involvement of dopamine D1 receptor and MEK signaling pathway in the ventromedial prefrontal cortex in consolidation and reconsolidation of recognition memory. Learn Mem 2009; 16: 243–247.
Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 2012; 151: 1126–1137.
Shimobayashi M, Hall MN . Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 2014; 15: 155–162.
Gerfen CR, Paletzki R, Heintz N . GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 2013; 80: 1368–1383.
Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA . Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci 2009; 32: 538–547.
Franklin K, Paxinos G . The Mouse Brain in Stereotaxic Coordinates, 3rd (edn). Elsevier: Cambridge, MA, USA, 2007.
Bertran-Gonzalez J, Hakansson K, Borgkvist A, Irinopoulou T, Brami-Cherrier K, Usiello A et al. Histone H3 phosphorylation is under the opposite tonic control of dopamine D2 and adenosine A2A receptors in striatopallidal neurons. Neuropsychopharmacology 2009; 34: 1710–1720.
Gangarossa G, Perroy J, Valjent E . Combinatorial topography and cell-type specific regulation of the ERK pathway by dopaminergic agonists in the mouse striatum. Brain Struct Funct 2013; 218: 405–419.
Gangarossa G, Espallergues J, de Kerchove d'Exaerde A, El Mestikawy S, Gerfen CR, Herve D et al. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits 2013; 7: 22.
Huynh TN, Santini E, Klann E . Requirement of Mammalian target of rapamycin complex 1 downstream effectors in cued fear memory reconsolidation and its persistence. J Neurosci 2014; 34: 9034–9039.
Santini E, Heiman M, Greengard P, Valjent E, Fisone G . Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Sci Signal 2009; 2: ra36.
Perez de la Mora M, Gallegos-Cari A, Crespo-Ramirez M, Marcellino D, Hansson AC, Fuxe K . Distribution of dopamine D(2)-like receptors in the rat amygdala and their role in the modulation of unconditioned fear and anxiety. Neuroscience 2012; 201: 252–266.
Parsons RG, Gafford GM, Helmstetter FJ . Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci 2006; 26: 12977–12983.
Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA 2005; 102: 491–496.
Biever A, Puighermanal E, Nishi A, David A, Panciatici C, Longueville S et al. PKA-dependent phosphorylation of ribosomal protein S6 does not correlate with translation efficiency in striatonigral and striatopallidal medium-sized spiny neurons. J Neurosci 2015; 35: 4113–4130.
Krawczyk M, Georges F, Sharma R, Mason X, Berthet A, Bezard E et al. Double-dissociation of the catecholaminergic modulation of synaptic transmission in the oval bed nucleus of the stria terminalis. J Neurophysiol 2011; 105: 145–153.
Dufner A, Thomas G . Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 1999; 253: 100–109.
Fadok JP, Darvas M, Dickerson TM, Palmiter RD . Long-term memory for pavlovian fear conditioning requires dopamine in the nucleus accumbens and basolateral amygdala. PLoS One 2010; 5: e12751.
Xu W, Sudhof TC . A neural circuit for memory specificity and generalization. Science 2013; 339: 1290–1295.
Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA . Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 2014; 17: 106–113.
Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S et al. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 2006; 9: 1028–1035.
Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B . Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 2013; 16: 332–339.
Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE . Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 2006; 26: 12387–12396.
LeDoux JE, Iwata J, Cicchetti P, Reis DJ . Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8: 2517–2529.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al. Distinct extended amygdala circuits for divergent motivational states. Nature 2013; 496: 224–228.
Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 2013; 496: 219–223.
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011; 471: 358–362.
Dong HW, Petrovich GD, Swanson LW . Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 2001; 38: 192–246.
Dong HW, Petrovich GD, Watts AG, Swanson LW . Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 2001; 436: 430–455.
Nagy FZ, Pare D . Timing of impulses from the central amygdala and bed nucleus of the stria terminalis to the brain stem. J Neurophysiol 2008; 100: 3429–3436.
Haufler D, Pare D . High-frequency oscillations are prominent in the extended amygdala. J Neurophysiol 2014; 112: 110–119.
Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S et al. A mesoscale connectome of the mouse brain. Nature 2014; 508: 207–214.
Bouthenet ML, Martres MP, Sales N, Schwartz JC . A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience 1987; 20: 117–155.
Krawczyk M, Sharma R, Mason X, Debacker J, Jones AA, Dumont EC . A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci 2011; 31: 8928–8935.
Naylor JC, Li Q, Kang-Park MH, Wilson WA, Kuhn C, Moore SD . Dopamine attenuates evoked inhibitory synaptic currents in central amygdala neurons. Eur J Neurosci 2010; 32: 1836–1842.
Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 2015; 519: 455–459.
Cai H, Haubensak W, Anthony TE, Anderson DJ . Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 2014; 17: 1240–1248.
Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci 2015; 18: 1493–1500.
Mahler SV, Berridge KC . Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 2009; 29: 6500–6513.
Blundell J, Kouser M, Powell CM . Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 2008; 90: 28–35.
Mac Callum PE, Hebert M, Adamec RE, Blundell J . Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory. Neurobiol Learn Mem 2014; 112: 176–185.
Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M . Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci USA 2011; 108: 3791–3796.
Acknowledgements
We thank members of the Valjent laboratory for discussions. We also thank Bo Li for insightful and constructive comments. This work was supported by Inserm (ATIP-Avenir), and the Agence Nationale de la Recherche (ANR-2010-JCJC-1412) to EV. DDB was a recipient of a postdoctoral fellowship from the Fondation pour la Recherche Médicale (FRM). J-AG is supported by an ERC Advanced Grant and is affiliated with the Paris School of Neuroscience and the Bio-Psy Laboratory of excellence.
Author contributions
DDB and EV designed the study. DDB conducted experiments and analyzed the data. CZ and JE assisted with the behavioral experiments. CRG and J-AG provided critical reagents and suggestions. DDB and EV wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
De Bundel, D., Zussy, C., Espallergues, J. et al. Dopamine D2 receptors gate generalization of conditioned threat responses through mTORC1 signaling in the extended amygdala. Mol Psychiatry 21, 1545–1553 (2016). https://doi.org/10.1038/mp.2015.210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.210
This article is cited by
-
Early life stress, prenatal secondhand smoke exposure, and the development of internalizing symptoms across childhood
Environmental Health (2023)
-
Nigrostriatal dopamine modulates the striatal-amygdala pathway in auditory fear conditioning
Nature Communications (2023)
-
Closed-loop brain stimulation augments fear extinction in male rats
Nature Communications (2023)
-
The effects of the recurrent social isolation stress on fear extinction and dopamine D2 receptors in the amygdala and the hippocampus
Pharmacological Reports (2023)
-
Dopaminergic innervation at the central nucleus of the amygdala reveals distinct topographically segregated regions
Brain Structure and Function (2023)