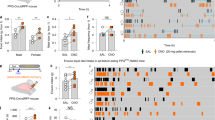
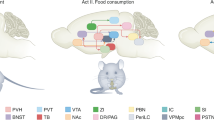
Abstract
For humans and animal models alike there is general agreement that the central nervous system processing of gastrointestinal (GI) signals arising from ingested food provides the principal determinant of the size of meals and their frequency. Despite this, relatively few studies are aimed at delineating the brain circuits, neurochemical pathways and intracellular signals that mediate GI-stimulation-induced intake inhibition. Two additional motivations to pursue these circuits and signals have recently arisen. First, the success of gastric-bypass surgery in obesity treatment is highlighting roles for GI signals such as glucagon-like peptide-1 (GLP-1) in intake and energy balance control. Second, accumulating data suggest that the intake-reducing effects of leptin may be mediated through an amplification of the intake-inhibitory effects of GI signals. Experiments reviewed show that: (1) the intake-suppressive effects of a peripherally administered GLP-1 receptor agonist is mediated by caudal brainstem neurons and that forebrain-hypothalamic neural processing is not necessary for this effect; (2) a population of medial nucleus tractus solitarius (NTS) neurons that are responsive to gastric distention is also driven by leptin; (3) caudal brainstem-targeted leptin amplifies the food-intake-inhibitory effects of gastric distention and intestinal nutrient stimulation; (4) adenosine monophosphate-activated protein kinase (AMPK) activity in NTS-enriched brain lysates is elevated by food deprivation and reduced by refeeding and (5) the intake-suppressive effect of hindbrain-directed leptin is reversed by elevating hindbrain AMPK activity. Overall, data support the view that the NTS and circuits within the hindbrain mediate the intake inhibition of GI signals, and that the effects of leptin on food intake result from the amplification of GI signal processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Smith GP . The direct and indirect controls of meal size. Neurosci Biobehav Rev 1996; 20: 41–46.
Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222: 339–350; discussion 350–352.
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Riddle MC, Henry RR, Poon TH, Zhang B, Mac SM, Holcombe JH et al. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes Metab Res Rev 2006; 22: 483–491.
Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8: 436–447.
Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N . Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol 1998; 275: R186–R193.
Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W . Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol 1998; 275: R180–R185.
Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR . Mode of action of OB protein (leptin) on feeding. Am J Physiol 1998; 275: R174–R179.
Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB . Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol 1997; 273: G920–G927.
Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL . Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005; 146: 3748–3756.
Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005; 1044: 127–131.
Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 1999; 276: R1541–R1544.
Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 1999; 44: 81–86.
Chelikani PK, Haver AC, Reidelberger RD . Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1695–R1706.
Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N et al. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes 1989; 38: 902–905.
Kreymann B, Williams G, Ghatei MA, Bloom SR . Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 1987; 2: 1300–1304.
Osaka T, Endo M, Yamakawa M, Inoue S . Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides 2005; 26: 1623–1631.
Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002; 110: 43–52.
Barragan JM, Eng J, Rodriguez R, Blazquez E . Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol 1999; 277: E784–E791.
Barragan JM, Rodriguez RE, Blazquez E . Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol 1994; 266: E459–E466.
Barragan JM, Rodriguez RE, Eng J, Blazquez E . Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept 1996; 67: 63–68.
Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C . Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997; 77: 257–270.
Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP . Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 1995; 7: 2294–2300.
Merchenthaler I, Lane M, Shughrue P . Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999; 403: 261–280.
Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006; 30: 1332–1340.
Norgren R . Projections from the nucleus of the solitary tract in the rat. Neuroscience 1978; 3: 207–218.
Norgren R, Leonard CM . Ascending central gustatory pathways. J Comp Neurol 1973; 150: 217–237.
Kinzig KP, D’Alessio DA, Seeley RJ . The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 2002; 22: 10470–10476.
Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V . Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1427–R1435.
Chaudhri OB, Parkinson JR, Kuo YT, Druce MR, Herlihy AH, Bell JD et al. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun 2006; 350: 298–306.
Kastin AJ, Akerstrom V . Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 2003; 27: 313–318.
Ma X, Bruning J, Ashcroft FM . Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci 2007; 27: 7125–7129.
Hayes MR, Skibicka KP, Grill HJ . Caudal brainstem processing is sufficient for behavioral, sympathetic and parasympathetic responses driven by peripheral and central glucagon-like-peptide-1 receptor stimulation. Endocrinology 2008; 149: 4059–4068.
Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 1993; 268: 19650–19655.
Sharma A, Sorenby A, Wernerson A, Efendic S, Kumagai-Braesch M, Tibell A . Exendin-4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia 2006; 49: 1247–1253.
Briones M, Bajaj M . Exenatide: a GLP-1 receptor agonist as novel therapy for Type 2 diabetes mellitus. Expert Opin Pharmacother 2006; 7: 1055–1064.
Doggrell SA . Recent evidence of sustained benefit with exenatide in Type 2 diabetes. Expert Opin Pharmacother 2006; 7: 2003–2006.
Yoo BK, Triller DM, Yoo DJ . Exenatide: a new option for the treatment of type 2 diabetes. Ann Pharmacother 2006; 40: 1777–1784.
Kaplan JM, Seeley RJ, Grill HJ . A behavioral probe of the growth of intake potential during the inter-meal interval in the rat. Behav Neurosci 1994; 108: 353–361.
Seeley RJ, Kaplan JM, Grill HJ . Effect of occluding the pylorus on intraoral intake: a test of the gastric hypothesis of meal termination. Physiol Behav 1995; 58: 245–249.
Hayes MR, Moore RL, Shah SM, Covasa M . 5-HT3 receptors participate in CCK-induced suppression of food intake by delaying gastric emptying. Am J Physiol Regul Integr Comp Physiol 2004; 287: R817–R823.
Baggio LL, Huang Q, Brown TJ, Drucker DJ . Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004; 127: 546–558.
Grill HJ, Kaplan JM . The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 2002; 23: 2–40.
McMahon LR, Wellman PJ . Decreased intake of a liquid diet in non food-deprived rats following intra-PVN injections of GLP-1 (7–36) amide. Pharmacol Biochem Behav 1997; 58: 673–677.
McMahon LR, Wellman PJ . PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 1998; 274: R23–R29.
Vrang N, Hansen M, Larsen PJ, Tang-Christensen M . Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 2007; 1149: 118–126.
Ahima RS, Saper CB, Flier JS, Elmquist JK . Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 2000; 21: 263–307.
Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ . Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med 2003; 9: 1539–1544.
Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006; 51: 801–810.
Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006; 51: 811–822.
Matson CA, Wiater MF, Kuijper JL, Weigle DS . Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides 1997; 18: 1275–1278.
Huo L, Maeng L, Bjorbaek C, Grill HJ . Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 2007; 148: 2189–2197.
Schwartz GJ, Moran TH . Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology 2002; 143: 3779–3784.
Hayes MR, Covasa M . Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res 2006; 1088: 120–130.
Willing AE, Berthoud HR . Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol 1997; 272: R59–R67.
Berthoud HR, Patterson LM . Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996; 156: 123–131.
Berthoud HR, Patterson LM, Neumann F, Neuhuber WL . Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997; 195: 183–191.
Vrang N, Phifer CB, Corkern MM, Berthoud HR . Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 2003; 285: R470–R478.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–574.
Xue B, Kahn BB . AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 2006; 574: 73–83.
Hayes MR, Skibicka KP, Bence KK, Grill HJ . Dorsal hindbrain AMP-Kinase as an intracellular mediator of energy balance. Endocrinology 2008; e-pub ahead of print 30 December 2008.
Acknowledgements
The work described was supported by NIH Grant DK-21397.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grill, H., Hayes, M. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes 33 (Suppl 1), S11–S15 (2009). https://doi.org/10.1038/ijo.2009.10
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.10
Keywords
This article is cited by
-
Hypothalamic endocannabinoids in obesity: an old story with new challenges
Cellular and Molecular Life Sciences (2021)
-
Afferents of the mouse linear nucleus
Molecular Brain (2020)
-
Timekeeping in the hindbrain: a multi-oscillatory circadian centre in the mouse dorsal vagal complex
Communications Biology (2020)
-
Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance
Neuropsychopharmacology (2018)
-
Neuronal connections of the central amygdalar nucleus with refeeding-activated brain areas in rats
Brain Structure and Function (2018)