Abstract
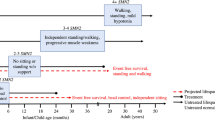
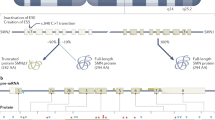
Cure SMA is dedicated to the treatment and cure of spinal muscular atrophy (SMA)—a disease affecting motor neurons, that robs patients of their ability to walk, eat and even breathe. Since 1984, we have directed and invested in comprehensive research that has shaped the scientific community’s understanding of SMA. On 23 December, 2016, the Food and Drug Administration (FDA) announced approval of Spinraza, a treatment developed by Biogen and Ionis, making it the first-ever approved therapy for SMA. Cure SMA provided early research funding in 2003 leading to the discovery of ISS-N1 sequence, now targeted by Spinraza. We are pleased that our strategy of providing seed funding for research to either identify new therapeutic strategies or de-risk early stage ones, has proven successful with Spinraza’s approval. The approval of Spinraza provides great hope to the SMA community and represents decades of hard work and perseverance by families, researchers, pharmaceutical companies and the FDA. Our hope is that Spinraza is the leading edge of a robust drug pipeline, and with our deep expertise in every aspect of SMA, we remain committed to do everything we can to support research and drug development to achieve the greatest possible effect for each and every SMA patient.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995; 80: 155–165.
Burghes AH, Beattie CE . Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci 2009; 10: 597–609.
Lorson CL, Hahnen E, Androphy EJ, Wirth B . A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci U S A 1999; 96: 6307–6311.
Cartegni L, Krainer AR . Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet 2002; 30: 377–384.
Singh NK, Singh NN, Androphy EJ, Singh RN . Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol 2006; 26: 1333–1346.
Hua Y, Vickers TA, Baker BF, Bennett CF, Krainer AR . Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol 2007; 5: e73.
Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 2016; 86: 890–897.
Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 2016; 388: 3017–3026.
Darras B, Swoboda K, Iannaccone S, Montes J, Castro D, Holuba N et al. Results of a phase 2 study of ISIS-SMNRx in children with spinal muscular atrophy. Neuromuscul Disord 2014; 24: 920.
Farrar MA, Park SB, Vucic S, Carey KA, Turner BJ, Gillingwater TH et al. Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol 2016; 81: 355–368.
Phan HC, Taylor JL, Hannon H, Howell R . Newborn screening for spinal muscular atrophy: anticipating an imminent need. Semin Perinatol 2015; 39: 217–229.
Bromberg MB, Swoboda KJ . Motor unit number estimation in infants and children with spinal muscular atrophy. Muscle Nerve 2002; 25: 445–447.
Le TT, McGovern VL, Alwine IE, Wang X, Massoni-Laporte A, Rich MM et al. Temporal requirement for high SMN expression in SMA mice. Hum Mol Genet 2011; 20: 3578–3591.
Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol 2016; 3: 132–145.
Lutz CM, Kariya S, Patruni S, Osborne MA, Liu D, Henderson CE et al. Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J Clin Invest 2011; 121: 3029–3041.
Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology 2014; 83: 810–817.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JG, ML, KH and JJ are employees of Cure SMA, and have no financial interest in this or any SMA drug program. Cure SMA has a financial interest in this and other SMA drug programs
Rights and permissions
About this article
Cite this article
Glascock, J., Lenz, M., Hobby, K. et al. Cure SMA and our patient community celebrate the first approved drug for SMA. Gene Ther 24, 498–500 (2017). https://doi.org/10.1038/gt.2017.39
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.39
This article is cited by
-
Current evidence for treatment with nusinersen for spinal muscular atrophy: a systematic review
Acta Neurologica Belgica (2019)
-
Spinal Muscular Atrophy Modeling and Treatment Advances by Induced Pluripotent Stem Cells Studies
Stem Cell Reviews and Reports (2019)