Abstract
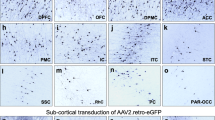
Dysfunction of the nigrostriatal system is the major cause of Parkinson's disease (PD). This brain region is therefore an important target for gene delivery aiming at disease modeling and gene therapy. Recombinant adeno-associated viral (rAAV) vectors have been developed as efficient vehicles for gene transfer into the central nervous system. Recently, several serotypes have been described, with varying tropism for brain transduction. In light of the further development of a viral vector-mediated rat model for PD, we performed a comprehensive comparison of the transduction and tropism for dopaminergic neurons (DNs) in the adult Wistar rat substantia nigra (SN) of seven rAAV vector serotypes (rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9). All vectors were normalized by titer and volume, and stereotactically injected into the SN. Gene expression was assessed non-invasively and quantitatively in vivo by bioluminescence imaging at 2 and 5 weeks after injection, and was found to be stable over time. Immunohistochemistry at 6 weeks following injection revealed the most widespread enhanced green fluorescence protein expression and the highest number of positive nigral cells using rAAV 2/7, 2/9 and 2/1. The area transduced by rAAV 2/8 was smaller, but nevertheless almost equal numbers of nigral cells were targeted. Detailed confocal analysis revealed that serotype 2/7, 2/9, 2/1 and 2/8 transduced at least 70% of the DNs. In conclusion, these results show that various rAAV serotypes efficiently transduce nigral DNs, but significant differences in transgene expression pattern and level were observed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jain S, Wood NW, Healy DG . Molecular genetic pathways in Parkinson's disease: a review. Clin Sci (Lond) 2005; 109: 355–364.
Lesage S, Brice A . Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet 2009; 18: R48–R59.
Gasser T . Mendelian forms of Parkinson's disease. Biochim Biophys Acta 2009; 1792: 587–596.
Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A . Genetics of Parkinson's disease and parkinsonism. Ann Neurol 2006; 60: 389–398.
Gasser T . Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med 2009; 11: e22.
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 2002; 22: 2780–2791.
Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ et al. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain 2007; 130: 799–815.
Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A . Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson's disease. Proc Natl Acad Sci USA 2003; 100: 2884–2889.
Lauwers E, Debyser Z, Van Dorpe J, De Strooper B, Nuttin B, Baekelandt V . Neuropathology and neurodegeneration in rodent brain induced by lentiviral vector-mediated overexpression of alpha-synuclein. Brain Pathol 2003; 13: 364–372.
Klein RL, King MA, Hamby ME, Meyer EM . Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum Gene Ther 2002; 13: 605–612.
Manfredsson FP, Burger C, Sullivan LF, Muzyczka N, Lewin AS, Mandel RJ . rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson's disease. Exp Neurol 2007; 207: 289–301.
Vercammen L, Van der Perren A, Vaudano E, Gijsbers R, Debyser Z, Van den Haute C et al. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson's disease. Mol Ther 2006; 14: 716–723.
Winklhofer KF . The parkin protein as a therapeutic target in Parkinson's disease. Expert Opin Ther Targets 2007; 11: 1543–1552.
Kirik D, Rosenblad C, Bjorklund A . Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol 1998; 152: 259–277.
Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the common marmoset. Neurosci Lett 1984; 50: 85–90.
Hoglinger GU, Oertel WH, Hirsch EC . The rotenone model of parkinsonism--the five years inspection. J Neural Transm Suppl 2006; 70: 269–272.
Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB Cory-Slechta DA . Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson's disease? Brain Res 2000; 873: 225–234.
Fleming SM, Fernagut PO, Chesselet MF . Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx 2005; 2: 495–503.
Freichel C, Neumann M, Ballard T, Muller V, Woolley M, Ozmen L et al. Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging 2007; 28: 1421–1435.
Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 2003; 278: 43628–43635.
Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet 2003; 12: 2277–2291.
Kahle PJ . alpha-Synucleinopathy models and human neuropathology: similarities and differences. Acta Neuropathol 2008; 115: 87–95.
Ulusoy A, Bjorklund T, Hermening S, Kirik D . In vivo gene delivery for development of mammalian models for Parkinson's disease. Exp Neurol 2008; 209: 89–100.
Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P . {alpha}-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci USA 2002; 99: 10813–10818.
Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci USA 2004; 101: 17510–17515.
Schneider B, Zufferey R, Aebischer P . Viral vectors, animal models and new therapies for Parkinson's disease. Parkinsonism Relat Disord 2008; 14 (Suppl 2): S169–S171.
Paterna JC, Feldon J, Bueler H . Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol 2004; 78: 6808–6817.
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 2004; 10: 302–317.
Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther 2007; 18: 195–206.
McFarland NR, Lee JS, Hyman BT, McLean PJ . Comparison of transduction efficiency of recombinant AAV serotypes 1, 2, 5, and 8 in the rat nigrostriatal system. J Neurochem 2009; 109: 838–845.
Dodiya HB, Bjorklund T, Stansell III J, Mandel RJ, Kirik D, Kordower JH . Differential transduction following basal ganglia administration of distinct pseudotyped AAV capsid serotypes in nonhuman primates. Mol Ther 2009; 18: 579–587.
Blits B, Derks S, Twisk J, Ehlert E, Prins J, Verhaagen J . Adeno-associated viral vector (AAV)-mediated gene transfer in the red nucleus of the adult rat brain: comparative analysis of the transduction properties of seven AAV serotypes and lentiviral vectors. J Neurosci Methods 2009; 185: 257–263.
Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA 2000; 97: 3428–3432.
Passini MA, Watson DJ, Wolfe JH . Gene delivery to the mouse brain with adeno-associated virus. Methods Mol Biol 2004; 246: 225–236.
Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM . Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 2004; 78: 6381–6388.
Vandenberghe LH, Breous E, Nam HJ, Gao G, Xiao R, Sandhu A et al. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Therapy 2009; 16: 1416–1428.
Vandenberghe LH, Wilson JM . AAV as an immunogen. Curr Gene Ther 2007; 7: 325–333.
Fitzsimons HL, Bland RJ, During MJ . Promoters and regulatory elements that improve adeno-associated virus transgene expression in the brain. Methods 2002; 28: 227–236.
Vandenberghe LH, Xiao R, Lock M, Lin J, Korn M, Wilson JM . Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther 2010; 21: 1251–1257.
Toelen J, VdP A, Carlon M, Michiels M, Lock M, Vandenberghe L et al. Gene Therapy submitted.
Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 2010; 21: 1259–1271.
Kugler S, Meyn L, Holzmuller H, Gerhardt E, Isenmann S, Schulz JB et al. Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors. Mol Cell Neurosci 2001; 17: 78–96.
Schoch S, Cibelli G, Thiel G . Neuron-specific gene expression of synapsin I. Major role of a negative regulatory mechanism. J Biol Chem 1996; 271: 3317–3323.
Grimm D, Kay MA . From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther 2003; 3: 281–304.
Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Therapy 2009; 17: 503–510.
Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP . AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 2008; 16: 89–96.
Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ . Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther 2007; 15: 1504–1511.
Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J et al. Comparative transduction efficiency of AAV vector serotypes 1-6 in the substantia nigra and striatum of the primate brain. Mol Ther 2010; 18: 588–593.
Flotte TR, Fischer AC, Goetzmann J, Mueller C, Cebotaru L, Yan Z et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol Ther 2010; 18: 594–600.
Prosch S, Stein J, Staak K, Liebenthal C, Volk HD, Kruger DH . Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol Chem Hoppe Seyler 1996; 377: 195–201.
Gao G, Vandenberghe LH, Wilson JM . New recombinant serotypes of AAV vectors. Curr Gene Ther 2005; 5: 285–297.
Ibrahimi A, Vande Velde G, Reumers V, Toelen J, Thiry I, Vandeputte C et al. Highly efficient multicistronic lentiviral vectors with peptide 2A sequences. Hum Gene Ther 2009; 20: 845–860.
Deroose CM, Reumers V, Gijsbers R, Bormans G, Debyser Z, Mortelmans L et al. Noninvasive monitoring of long-term lentiviral vector-mediated gene expression in rodent brain with bioluminescence imaging. Mol Ther 2006; 14: 423–431.
Baekelandt V, Claeys A, Eggermont K, Lauwers E, De Strooper B, Nuttin B et al. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum Gene Ther 2002; 13: 841–853.
Schmitz C, Hof PR . Design-based stereology in neuroscience. Neuroscience 2005; 130: 813–831.
Acknowledgements
We thank Martine Michiels and Phebe van Wijk for their excellent technical assistance. AVdP, JT and MC are doctoral fellows supported by grants from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). BH is a doctoral fellow supported by a grant from the Flemish Fund for Scientific Research (FWO-Vlaanderen). Research was funded by the IWT-Vlaanderen (IWT SBO/060838 and IWT SBO/80020), by the EC-FP6 program ‘DiMI’ (LSHB-CT-2005-512146), the FP7 RTD project MEFOPA (HEALTH-2009-241791), the KULeuven (IOF-KP/07/001 and OT/08/052A), the Interuniversity Attraction Pole programme NiMI (P6/38) and the KULeuven Center of Excellence ‘MoSAIC’ (EF/05/08). Confocal images were taken in the cell imaging core of the KULeuven.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LHV holds patents on the technology described within. JMW is an inventor on patents licensed to various biopharmaceutical companies, including ReGenX, for which he has equity in, consults for and receives a grant from.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Van der Perren, A., Toelen, J., Carlon, M. et al. Efficient and stable transduction of dopaminergic neurons in rat substantia nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther 18, 517–527 (2011). https://doi.org/10.1038/gt.2010.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.179
Keywords
This article is cited by
-
LRRK2 Ablation Attenuates Alpha-Synuclein–Induced Neuroinflammation Without Affecting Neurodegeneration or Neuropathology In Vivo
Neurotherapeutics (2021)
-
The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies
Acta Neuropathologica (2020)
-
AAV2/DJ-mediated alpha-synuclein overexpression in the rat substantia nigra as early stage model of Parkinson’s disease
Cell and Tissue Research (2019)
-
In vivo models of alpha-synuclein transmission and propagation
Cell and Tissue Research (2018)
-
Transient and localized optogenetic activation of somatostatin-interneurons in mouse visual cortex abolishes long-term cortical plasticity due to vision loss
Brain Structure and Function (2018)