Abstract
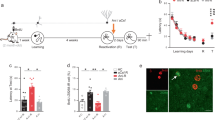
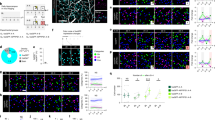
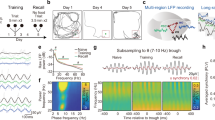
Retrograde amnesia observed following hippocampal lesions in humans and animals is typically temporally graded1,2, with recent memory being impaired while remote memories remain intact, indicating that the hippocampal formation has a time-limited role in memory storage3,4. However, this claim remains controversial because studies involving hippocampal lesions tell us nothing about the contribution of the hippocampus to memory storage if this region was present at the time of memory retrieval5,6. We therefore used non-invasive functional brain imaging using (14C)2-deoxyglucose uptake to examine how the brain circuitry underlying long-term memory storage is reorganized over time in an intact brain. Regional metabolic activity in the brain was mapped in mice tested at different times for retention of a spatial discrimination task. Here we report that increasing the retention interval from 5 days to 25 days resulted in both decreased hippocampal metabolic activity during retention testing and a loss of correlation between hippocampal metabolic activity and memory performance. Concomitantly, a recruitment of certain cortical areas was observed. These results indicate that there is a time-dependent reorganization of the neuronal circuitry underlying long-term memory storage, in which a transitory interaction between the hippocampal formation and the neocortex would mediate the establishment of long-lived cortical memory representations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reed, J. M. & Squire, L. R. Retrograde amnesia for facts and events: findings from four new cases. J. Neurosci. 18, 3943–3954 (1998).
Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys and humans. Psychol. Rev. 99, 195–231 (1992).
Zola-Morgan, S. M. & Squire, L. R. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science 250, 288–290 (1990).
Anagnostaras, S. G., Maren, S. & Fanselow, M. S. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J. Neurosci. 19, 1106–1114 (1999).
Moscovitch, M. & Nadel, L. Consolidation and the hippocampal complex revisited: in defense of the multiple-trace model. Curr. Opin. Neurobiol. 8, 297–300 (1998).
Knowlton, B. J. & Fanselow, M. S. The hippocampus, consolidation and on-line memory. Curr. Opin. Neurobiol. 8, 293–296 (1998).
Polster, M. R., Nadel, L. & Schacter, D. L. Cognitive neuroscience analysis of memory: a historical perspective. J. Cogn. Neurosci. 3, 95–116 (1991).
Dudai, Y. Consolidation: fragility on the road to the engram. Neuron 17, 367–370 (1996).
Squire, L. R. & Alvarez, P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 5, 169–177 (1995).
Sokoloff, L.et al. The 14C-deoxyglucose method for measurement of local cerebral glucose utilization: theory, procedure and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28, 897–916 (1977).
Bontempi, B., Jaffard, R. & Destrade, C. Differential temporal evolution of post-training changes in regional brain glucose metabolism induced by repeated spatial discrimination training in mice: visualization of the memory consolidation process? Eur. J. Neurosci. 8, 2348–2360 (1996).
Cho, Y. H., Beracochea, D. & Jaffard, R. Extended temporal gradient for the retrograde and anterograde amnesia produced by ibotenate entorhinal cortex lesions in mice. J. Neurosci. 13, 1759–1766 (1993).
Finch, D. M., Derian, E. L. & Babb, T. L. Afferent fibers to rat cingulate cortex. Exp. Neurol. 83, 468–485 (1984).
Sif, J., Meunier, M., Messier, C., Calas, A. & Destrade, C. Quantitative (14C)2-deoxyglucose study of a functional dissociation between anterior and posterior cingulate cortices in mice. Neurosci. Lett. 101, 223–228 (1989).
Nyberg, L.et al. Network analysis of positron emission tomography regional cerebral blood flow data: ensemble inhibition during episodic memory retrieval. J. Neurosci. 16, 3753–3759 (1996).
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 100, 147–154 (1986).
Damasio, A. R. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition 33, 25–62 (1989).
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford Univ. Press, 1978).
Meunier, M., Jaffard, R. & Destrade, C. Differential involvement of anterior and posterior cingulate cortices in spatial discrimination learning in a T-maze in mice. Behav. Brain Res. 44, 133–143 (1991).
Markowitsch, H. J. Which brain regions are critically involved in the retrieval of old episodic memory. Brain Res. Rev. 21, 117–127 (1995).
Buckner, R. L., Kelley, W. M. & Petersen, S. E. Frontal cortex contributes to human memory formation. Nature Neurosci. 2, 311–314 (1999).
Fink, G. R.et al. Cerebral representation of one's own past: neural networks involved in autobiographical memory. J. Neurosci. 16, 4275–4282 (1996).
Ungerleider, L. G. Functional brain imaging studies of cortical mechanisms for memory. Science 270, 769–775 (1995).
Schacter, D. L., Alpert, N. M., Savage, C. R., Rauch, S. L. & Albert, M. S. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc. Natl Acad. Sci. USA 93, 321–325 (1996).
Gabrieli, J. D. E. Cognitive neuroscience of human memory. Annu. Rev. Psychol. 49, 87–115 (1998).
Alkire, M. T., Haier, R. J., Fallon, J. H. & Cahill, L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proc. Natl Acad. Sci. USA 95, 14506–14510 (1998).
Buzsaki, G. Memory consolidation during sleep: a neuropsychological perspective. J. Sleep Res. 7, 17–23 (1998).
Lehman, A. Atlas Stéréotaxique du Cerveau de la Souris (Editions du CNRS, Paris, 1974).
McIntosh, A. R. & Gonzalez-Lima, F. Network interactions among limbic cortices, basal forebrain and cerebellum differentiate a tone conditioned as a Pavlovian excitor or inhibitor: fluorodeoxyglucose mapping and covariance structural modelling. J. Neurophysiol. 72, 1717–1733 (1994).
Acknowledgements
We thank T. Durkin, B. Poucet and Y. H. Cho for comments on earlier drafts of the manuscript. This work was funded by grants from the CNRS and the Université Bordeaux I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bontempi, B., Laurent-Demir, C., Destrade, C. et al. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400, 671–675 (1999). https://doi.org/10.1038/23270
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/23270
This article is cited by
-
Replay, the default mode network and the cascaded memory systems model
Nature Reviews Neuroscience (2022)
-
Cortical thickness across the cingulate gyrus in schizophrenia and its association to illness duration and memory performance
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
Retrosplenial cortex in spatial memory: focus on immediate early genes mapping
Molecular Brain (2021)
-
Neurochemical mechanisms for memory processing during sleep: basic findings in humans and neuropsychiatric implications
Neuropsychopharmacology (2020)
-
Flexible use of allocentric and egocentric spatial memories activates differential neural networks in mice
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.