Abstract
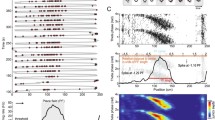
The phase relationship between the activity of hippocampal place cells and the hippocampal theta rhythm systematically precesses as the animal runs through the region in an environment called the place field of the cell. We present a minimal biophysical model of the phase precession of place cells in region CA3 of the hippocampus. The model describes the dynamics of two coupled point neurons—namely, a pyramidal cell and an interneuron, the latter of which is driven by a pacemaker input. Outside of the place field, the network displays a stable, background firing pattern that is locked to the theta rhythm. The pacemaker input drives the interneuron, which in turn activates the pyramidal cell. A single stimulus to the pyramidal cell from the dentate gyrus, simulating entrance into the place field, reorganizes the functional roles of the cells in the network for a number of cycles of the theta rhythm. In the reorganized network, the pyramidal cell drives the interneuron at a higher frequency than the theta frequency, thus causing a systematic precession relative to the theta input. The frequency of the pyramidal cell can vary to account for changes in the animal's running speed. The transient dynamics end after up to 360 degrees of phase precession when the pacemaker input to the interneuron occurs at a phase to return the network to the stable background firing pattern, thus signaling the end of the place field. Our model, in contrast to others, reports that phase precession is a temporally, and not spatially, controlled process. We also predict that like pyramidal cells, interneurons phase precess. Our model provides a mechanism for shutting off place cell firing after the animal has crossed the place field, and it explains the observed nearly 360 degrees of phase precession. We also describe how this model is consistent with a proposed autoassociative memory role of the CA3 region.
Similar content being viewed by others
References
Bullock TH, Buzsaki G, McClune MC (1990) Coherence of compound field potentials reveals discontinuities in the calsubiculum of the hippocampus in freely-moving rats. Neurosci. 38:609-619.
Buzsáki G, Eidelberg E (1982) Direct afferent excitation and long term potentiation of hippocampal interneurons. J. Neurophysiol. 48:597-607.
Csicsvari J, Hirase H, Czurko A, Buzsaki G (1998) Reliability and state-dependence of pyramidal cell-interneuron synapses in the hippocampus: An ensemble approach in the behaving rat. Neuron 21:179-189.
Ermentrout GB, Kopell N (1998) Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc. Natl. Acad. Sci. 95:1259-1264.
Fox SE, Ranck JB (1975) Localization and anatomical identification of theta and complex spike cells in dorsal hippocampal formation of rats. Exp. Neurol. 49:299-313.
Fox SE, Wolfson S, Ranck JB (1986) Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Exp. Brain Res. 62:495-500.
Freund TF, Antal M (1988) GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336:170-173.
Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347-470.
Gardner-Medwin AR (1976) The recall of events through the learning of associations between their parts. Proc. R. Soc. Lond. B 194:375-402.
Gibson WG, Robinson J (1992) Statistical analysis of the dynamics of a sparse associative memory. Neural Networks 5:645-661.
Green J, Arduini A (1954) Hippocampal electrical activity in arousal. J. Neurophysiol. 17:533-557.
Hirase H, Recce M (1996) A search for the optimal thresholding sequence in an associative memory. Network 7:741-756.
Jensen O, Lisman JE (1996) Hippocampal CA3 region predicts memory sequences: Accounting for the phase advance of place cells. Learn. Mem. 3:257-263.
Jung MW, McNaughton BL (1993) Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3:165-182.
Kamondi A, Acsady L, Wang X, Buzsaki G (1998) Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: Activity-dependent phase-precession of action potentials. Hippocampus 8:244-261.
King C, Recce M, O'Keefe J (1998) The rhythmicity of cells of the medial septum/diagonal band of broca in the awake freely moving rat: Relationship with behaviour and hippocampal theta. Eur. J. Neurosci. 10:464-467.
Kopell N, Somers D (1995) Anti-phase solutions in relaxation oscillators coupled through excitatory interactions. J. Math. Biol. 33:261-280.
Marr D (1971) Simple memory: A theory for archicortex. Phil. Trans. R. Soc. Lond. B 176:23-81.
Mishchenko EF, Rozov NK (1980) Differential Equations with Small Parameters and Relaxation Oscillators. Plenum Press, New York.
Morris C, Lecar H (1981) Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 35:193-213.
Muller RU, Kubie JL, Bostock EM, Taube JS, Quirk G (1987) Spatial firing correlates of neurons in the hippocampal formation of freely moving rats. J. Neurosci. 7:1951-1968.
Nadim F, Manor Y, Nussbaum P, Marder E (1998) Frequency regulation of a slow rhythm by a fast periodic input. J. Neurosci. 18:5053-5067.
O'Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp. Neurol. 51:78-109.
O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely moving rat. Brain Research 34:171-175.
O'Keefe J, Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3:317-330.
Recce M (1994) The representation of space in the rat hippocampus. Ph.D. thesis, University College London.
Recce M (1999) Encoding information in neuronal activity. In: Maass W, Bishop C, eds. Pulsed Neural Networks. MIT Press, Cambridge, MA. pp. 111-131.
Recce M, Harris KD (1996) Memory for places: A navigational model in support of Marr's theory of hippocampal function. Hippocampus 6:735-748.
Rieke F, Warland D, de Ruyter van Steveninck D, Bialek W (1997) Spikes-Exploring the Neural Code. MIT Press, Cambridge, MA.
Rinzel J, Ermentrout G (1998) In: Koch C, Segev I, eds. Methods in Neuronal Modeling: From analysis of Neural Excibability and Oscillations to Networks. 2nd edition. MIT Press, Cambridge, MA. pp. 251-291.
Rubin J, Terman D (2000) Geometric analysis of population rhythms in synaptically coupled neuronal networks. Neur. Comp., 12:597-645.
Skaggs WE, McNaughton BL, Wilson MA, Barnes CA (1996) Theta-phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6:149-172.
Somers D, Kopell N (1993) Rapid synchronization through fast threshold modulation. Biol. Cyber. 68:393-407.
Terman D, Kopell N, Bose A (1998) Dynamics of two mutually coupled slow inhibitory neurons. Physica D 117:241-275.
Terman D, Lee E (1997) Partial synchronization in a network of neural oscillators. SIAM J. Appl. Math. 57:252-293.
Treves A, Rolls ET (1994) A computational analysis of the role of the hippocampus in memory. Hippocampus 4:373-391.
Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL (1996) Population dynamics and theta rhythm phase precession of hippocampal place cell firing: A spiking neuron model. Hippocampus 6:271-280.
van Vreeswijk C, Abbott L, Ermentrout GB (1994) When inhibition, not excitation synchronizes neural firing. J. Comp. Neurosci. 1:313-321.
Wallenstein GV, Hasselmo ME (1997) GABAergic modulation of hippocampal population activity: Sequence learning, place field development, and the phase precession effect. J. Neurophysiol. 78:393-408.
Wang XJ, Rinzel J (1992) Alternating and synchronous rhythms in reciprocally inhibitory neuron models. Neural Comp. 4:84-97.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bose, A., Booth, V. & Recce, M. A Temporal Mechanism for Generating the Phase Precession of Hippocampal Place Cells. J Comput Neurosci 9, 5–30 (2000). https://doi.org/10.1023/A:1008976210366
Issue Date:
DOI: https://doi.org/10.1023/A:1008976210366