Abstract
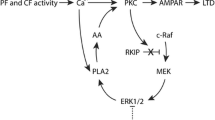
Protein kinase C (PKC), a family of serine/threonine protein kinases, mediates a myriad of patho-physiological cellular events in various tissues. The originally discovered PKC (conventional) requires the binding of diacylglycerol and Ca2+ for full activation. The conventional PKC consists of four isoforms, PKCα, PKCβI/βII, and PKCγ. PKCα and PKCβI/βII are expressed in the cells of various tissues including the brain, while PKCγ is present specifically in neurons of the brain and spinal cord. The cerebellum expresses the largest amount of PKC with all its four isoforms. Purkinje cells express PKCα and PKCγ. Previous studies have shown that PKCα is involved in the induction of long-term depression (LTD) at parallel fiber–Purkinje cell synapses. On the other hand, analysis of PKCγ-deficient mice has revealed that PKCγ plays a critical role in eliminating supernumerary climbing fiber synapses from developing Purkinje cells. Although why PKCα has no compensatory action in climbing fiber pruning in PKCγ-deficient Purkinje cells had so far remained unclear, we have recently demonstrated that PKCα is also capable of pruning supernumerary climbing fiber synapses, but the expression levels of PKCα are too low to achieve pruning in PKCγ-null Purkinje cells. Notably, although PKCγ is most abundant in Purkinje cells, its physiological role in mature Purkinje cells remained totally unknown. In addition to a concise review of the physiological and pathological roles of conventional PKCs in Purkinje cells, this report postulates a contribution of PKCα in developing Purkinje cells and a possible involvement of PKCγ in motor coordination in the mature cerebellum.



Similar content being viewed by others
References
Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–6.
Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–9.
Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979;254:3692–5.
Kishimoto A, Takai Y, Mori T, Kikkawa U, Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980;255:2273–6.
Kaibuchi K, Sano K, Hoshijima M, Takai Y, Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982;3:323–35.
Nishizuka Y. Studies and perspectives of protein kinase C. Science (New York, NY). 1986;233:305–12.
Ase K, Saito N, Shearman MS, Kikkawa U, Ono Y, Igarashi K, et al. Distinct cellular expression of beta I- and beta II-subspecies of protein kinase C in rat cerebellum. J Neurosci Off J Soc Neurosci. 1988;8:3850–6.
Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–96.
Takahashi N, Shuvaev AN, Konno A, Matsuzaki Y, Watanave M, Hirai H. Regulatory connection between the expression level of classical protein kinase C and pruning of climbing fibers from cerebellar Purkinje cells. J Neurochem. 2017; https://doi.org/10.1111/jnc.14239.
Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–87.
Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34:1697–710. https://doi.org/10.1111/j.1460-9568.2011.07894.x.
Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, et al. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell. 1995;83:1223–31.
Kano M, Hashimoto K, Tabata T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos Trans R Soc Lond Ser B Biol Sci. 2008;363:2173–86. https://doi.org/10.1098/rstb.2008.2270.
Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, et al. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. 1995;83:1233–42.
Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–8.
Leitges M, Kovac J, Plomann M, Linden DJ. A unique PDZ ligand in PKCalpha confers induction of cerebellar long-term synaptic depression. Neuron. 2004;44:585–94. https://doi.org/10.1016/j.neuron.2004.10.024.
Hanley JG. PICK1: a multi-talented modulator of AMPA receptor trafficking. Pharmacol Ther. 2008;118:152–60. https://doi.org/10.1016/j.pharmthera.2008.02.002.
Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–84. https://doi.org/10.1038/386279a0.
Hirai H. Modification of AMPA receptor clustering regulates cerebellar synaptic plasticity. Neurosci Res. 2001;39:261–7.
Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci Off J Soc Neurosci. 2000;20:7258–67.
Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–74. https://doi.org/10.1093/emboj/19.12.2765.
Shuvaev AN, Horiuchi H, Seki T, Goenawan H, Irie T, Iizuka A, et al. Mutant PKCgamma in spinocerebellar ataxia type 14 disrupts synapse elimination and long-term depression in Purkinje cells in vivo. J Neurosci Off J Soc Neurosci. 2011;31:14324–34. https://doi.org/10.1523/jneurosci.5530-10.2011.
Schrenk K, Kapfhammer JP, Metzger F. Altered dendritic development of cerebellar Purkinje cells in slice cultures from protein kinase Cgamma-deficient mice. Neuroscience. 2002;110:675–89.
Gundlfinger A, Kapfhammer JP, Kruse F, Leitges M, Metzger F. Different regulation of Purkinje cell dendritic development in cerebellar slice cultures by protein kinase Calpha and -beta. J Neurobiol. 2003;57:95–109. https://doi.org/10.1002/neu.10259.
Shutoh F, Katoh A, Ohki M, Itohara S, Tonegawa S, Nagao S. Role of protein kinase C family in the cerebellum-dependent adaptive learning of horizontal optokinetic response eye movements in mice. Eur J Neurosci. 2003;18:134–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hirai, H. Protein Kinase C in the Cerebellum: Its Significance and Remaining Conundrums. Cerebellum 17, 23–27 (2018). https://doi.org/10.1007/s12311-017-0898-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0898-x