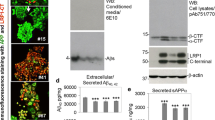
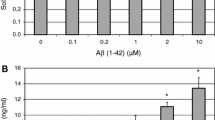
Abstract
Our lab has recently shown that the Sigma-2 Receptor/Transmembrane Protein 97 (TMEM97) and Progesterone Receptor Membrane Component 1 (PGRMC1) form a complex with the Low Density Lipoprotein Receptor (LDLR), and this intact complex is required for efficient uptake of lipoproteins such as LDL and apolipoprotein E (apoE). These receptors are expressed in the nervous system where they have implications in neurodegenerative diseases such as Alzheimer’s disease (AD), where apoE is involved in neuronal uptake and accumulation of Aβ42, eventually cascading into neurodegeneration, synaptic dysfunction, and ultimately, dementia. We hypothesize that the intact Sigma-2 receptor complex—TMEM97, PGRMC1, and LDLR—is necessary for internalization of apoE and Aβ42 monomers (mAβ42) and oligomers (oAβ42), and the disruption of the receptor complex inhibits uptake. The results of this study suggest that the intact Sigma-2 receptor complex is a binding site for mAβ42 and oAβ42, in the presence or absence of apoE2, apoE3, and apoE4. The loss or pharmacological inhibition of one or both of these proteins results in the disruption of the complex leading to decreased uptake of mAβ42 and oAβ42 and apoE in primary neurons. The TMEM97, PGRMC1, and LDLR complex is a pathway for the cellular uptake of Aβ42 via apoE dependent and independent mechanisms. This study suggests that the complex may potentially be a novel pharmacological target to decrease neuronal Aβ42 internalization and accumulation, which may represent a new strategy for inhibiting the rate of neurotoxicity, neurodegeneration, and progression of AD.





Similar content being viewed by others
Data Availability
Not applicable
Abbreviations
- Aβ42:
-
Amyloid beta 1–42
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- apoE:
-
Apolipoprotein E
- CNS:
-
Central nervous system
- DKO:
-
Double knockout
- DMPC:
-
Dimyristoyl-phosphatidylcholine
- fAβ42:
-
Aβ42 fibrils
- HFIP:
-
Hexafluoroisopropanol
- LDL:
-
Low density lipoprotein
- LDLR:
-
Low density lipoprotein receptor
- LPDS:
-
Lipoprotein depleted serum
- LRP:
-
LDLR-related protein
- mAβ42:
-
Aβ42 monomers
- oAβ42:
-
Aβ42 oligomers
- PBS:
-
Phosphate-buffered saline
- PBS-T:
-
Phosphate-buffered saline with 0.2% Tween 20
- PGRMC1:
-
Progesterone receptor membrane component 1
- PLL:
-
Poly L-lysine
- TEM:
-
Transmission electron microscopy
- TMEM97:
-
Transmembrane protein
References
Bowen WD (2000) Sigma receptors: recent advances and new clinical potentials. Pharm Acta Helv 74:211–218
Mach RH, Zeng C, Hawkins WG (2013) The sigma2 receptor: a novel protein for the imaging and treatment of cancer. J Med Chem 56:7137–7160. https://doi.org/10.1021/jm301545c
Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC (1990) Sigma receptors: biology and function. Pharmacol Rev 42:355–402
Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE (1976) The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197:517–532
Leonard BE (2004) Sigma receptors and sigma ligands: background to a pharmacological enigma. Pharmacopsychiatry 37(Suppl 3):S166–S170. https://doi.org/10.1055/s-2004-832674
Izzo NJ, Staniszewski A, To L, Fa M, Teich AF, Saeed F, Wostein H, Walko T 3rd et al (2014) Alzheimer's therapeutics targeting amyloid beta 1–42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS One 9:e111898. https://doi.org/10.1371/journal.pone.0111898
Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, Rehak C, Yurko R et al (2014) Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One 9:e111899. https://doi.org/10.1371/journal.pone.0111899
Riad A, Zeng C, Weng CC, Winters H, Xu K, Makvandi M, Metz T, Carlin S et al (2018) Sigma-2 receptor/TMEM97 and PGRMC-1 increase the rate of internalization of LDL by LDL receptor through the formation of a ternary complex. Sci Rep 8:16845. https://doi.org/10.1038/s41598-018-35430-3
Butterfield DA (2002) Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res 36:1307–1313. https://doi.org/10.1080/1071576021000049890
Findeis MA (2007) The role of amyloid beta peptide 42 in Alzheimer's disease. Pharmacol Ther 116:266–286. https://doi.org/10.1016/j.pharmthera.2007.06.006
Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL (1989) Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science 245:417–420. https://doi.org/10.1126/science.2474201
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277:32046–32053. https://doi.org/10.1074/jbc.M201750200
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82:4245–4249. https://doi.org/10.1073/pnas.82.12.4245
Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J et al (2004) Abeta localization in abnormal endosomes: Association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging 25:1263–1272. https://doi.org/10.1016/j.neurobiolaging.2004.02.027
D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH (2001) Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology 38:120–134. https://doi.org/10.1046/j.1365-2559.2001.01082.x
Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V et al (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156:15–20. https://doi.org/10.1016/s0002-9440(10)64700-1
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 46:860–866. https://doi.org/10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m
Friedrich RP, Tepper K, Ronicke R, Soom M, Westermann M, Reymann K, Kaether C, Fandrich M (2010) Mechanism of amyloid plaque formation suggests an intracellular basis of Abeta pathogenicity. Proc Natl Acad Sci U S A 107:1942–1947. https://doi.org/10.1073/pnas.0904532106
Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM (2009) Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci U S A 106:20324–20329. https://doi.org/10.1073/pnas.0911281106
Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E (2010) Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol 119:523–541. https://doi.org/10.1007/s00401-010-0679-9
Domert J, Rao SB, Agholme L, Brorsson AC, Marcusson J, Hallbeck M, Nath S (2014) Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol Dis 65:82–92. https://doi.org/10.1016/j.nbd.2013.12.019
Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M (2012) Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci 32:8767–8777. https://doi.org/10.1523/JNEUROSCI.0615-12.2012
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103:11172–11177. https://doi.org/10.1073/pnas.0603838103
Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M (2018) Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136:41–56. https://doi.org/10.1007/s00401-018-1868-1
Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R (2010) Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci 13:190–196. https://doi.org/10.1038/nn.2476
Mahley RW, Rall SC Jr (2000) Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1:507–537. https://doi.org/10.1146/annurev.genom.1.1.507
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10:333–344. https://doi.org/10.1038/nrn2620
Verghese PB, Castellano JM, Holtzman DM (2011) Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 10:241–252. https://doi.org/10.1016/S1474-4422(10)70325-2
Wisniewski T, Frangione B (1992) Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 135:235–238. https://doi.org/10.1016/0304-3940(92)90444-c
Hou C, Tu Z, Mach R, Kung HF, Kung MP (2006) Characterization of a novel iodinated sigma-2 receptor ligand as a cell proliferation marker. Nucl Med Biol 33:203–209. https://doi.org/10.1016/j.nucmedbio.2005.10.001
Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RH (2005) [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl]-2-methoxy-5-methyl benzamide: a novel sigma-2 receptor probe. Eur J Pharmacol 525:8–17. https://doi.org/10.1016/j.ejphar.2005.09.063
Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16:109–120. https://doi.org/10.1038/nrn3887
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM et al (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141:2181–2193. https://doi.org/10.1093/brain/awy146
Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, Xie SX, McBride J et al (2014) A platform for discovery: the University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement 10:477–484.e1. https://doi.org/10.1016/j.jalz.2013.06.003
Xiao Y, Ma B, McElheny D, Parthasarathy S, Long F, Hoshi M, Nussinov R, Ishii Y (2015) Abeta(1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer's disease. Nat Struct Mol Biol 22:499–505. https://doi.org/10.1038/nsmb.2991
Kuipers BJ, Gruppen H (2007) Prediction of molar extinction coefficients of proteins and peptides using UV absorption of the constituent amino acids at 214 nm to enable quantitative reverse phase high-performance liquid chromatography-mass spectrometry analysis. J Agric Food Chem 55:5445–5451. https://doi.org/10.1021/jf070337l
Stine WB, Jungbauer L, Yu C, LaDu MJ (2011) Preparing synthetic Abeta in different aggregation states. Methods Mol Biol 670:13–32. https://doi.org/10.1007/978-1-60761-744-0_2
Innerarity TL, Pitas RE, Mahley RW (1986) Lipoprotein-receptor interactions. Methods Enzymol 129:542–565. https://doi.org/10.1016/0076-6879(86)29091-6
Zeng C, Weng CC, Schneider ME Jr, Puentes L, Riad A, Xu K, Makvandi M, Jin L et al (2019) TMEM97 and PGRMC1 do not mediate sigma-2 ligand-induced cell death. Cell Death Discov 5:58. https://doi.org/10.1038/s41420-019-0141-2
Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19:8876–8884
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95:6448–6453. https://doi.org/10.1073/pnas.95.11.6448
Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–118. https://doi.org/10.1038/nrneurol.2012.263
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179:312–339. https://doi.org/10.1016/j.cell.2019.09.001
Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M et al (1994) Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci U S A 91:11183–11186. https://doi.org/10.1073/pnas.91.23.11183
Gylys KH, Fein JA, Tan AM, Cole GM (2003) Apolipoprotein E enhances uptake of soluble but not aggregated amyloid-beta protein into synaptic terminals. J Neurochem 84:1442–1451. https://doi.org/10.1046/j.1471-4159.2003.01643.x
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H et al (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11:457–470. https://doi.org/10.1038/nrneurol.2015.119
Zeng C, Garg N, Mach RH (2016) The PGRMC1 protein level correlates with the binding activity of a Sigma-2 fluorescent probe (SW120) in rat brain cells. Mol Imaging Biol 18:172–179. https://doi.org/10.1007/s11307-015-0891-z
Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC (2000) Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in beta-amyloid precursor protein-transgenic mice. J Neurosci 20:3606–3611
Fuentealba RA, Liu Q, Zhang J, Kanekiyo T, Hu X, Lee JM, LaDu MJ, Bu G (2010) Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One 5:e11884. https://doi.org/10.1371/journal.pone.0011884
Funding
Research was supported by the Michael J Fox Foundation and NIH NIDA T32 Fellowship.
Author information
Authors and Affiliations
Contributions
AR performed and analyzed uptake experiments in HeLa cells and primary rat neurons, ELISA analysis, confocal microscopy experiments, and manuscript preparation. ZL performed and analyzed TEM experiments and Aβ42 fibril preparations, CZ performed CRISPR knockout of HeLa cell lines and Aβ42 microscopy on human tissues, CW performed and analyzed radioligand binding experiments, VL and JT were responsible for tissue sample acquisition, analysis, and pathology, RHM was responsible for experimental organization and rationale, data analysis, organization, and manuscript preparation.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
All human tissue was used in accordance with the University of Pennsylvania IRB protocol.
Consent for Publication
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 83438 kb)
Rights and permissions
About this article
Cite this article
Riad, A., Lengyel-Zhand, Z., Zeng, C. et al. The Sigma-2 Receptor/TMEM97, PGRMC1, and LDL Receptor Complex Are Responsible for the Cellular Uptake of Aβ42 and Its Protein Aggregates. Mol Neurobiol 57, 3803–3813 (2020). https://doi.org/10.1007/s12035-020-01988-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01988-1