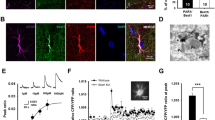
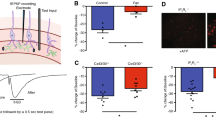
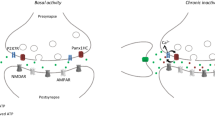
Abstract
A key feature of neurotransmission is its ability to adapt to changes in neuronal environment, which is essential for many brain functions. Homeostatic synaptic plasticity (HSP) emerges as a compensatory mechanism used by neurons to adjust their excitability in response to changes in synaptic activity. Recently, glial cells emerged as modulators for neurotransmission by releasing gliotransmitters into the synaptic cleft through pathways that include P2X7 receptors (P2X7R), connexons, and pannexons. However, the role of gliotransmission in the activity-dependent adjustment of presynaptic strength is still an open question. Here, we investigated whether glial cells participate in HSP upon chronic inactivity and the role of adenosine triphosphate (ATP), connexin43 hemichannels (Cx43HCs), and pannexin1 (Panx1) channels in this process. We used immunocytochemistry against vesicular glutamate transporter 1 (vGlut1) to estimate changes in synaptic strength in hippocampal dissociated cultures. Pharmacological manipulations indicate that glial-derived ATP and P2X7R are required for HSP. In addition, inhibition of Cx43 and Panx1 channels reveals a pivotal role for these channels in the compensatory adjustment of synaptic strength, emerging as new pathways for ATP release upon inactivity. The involvement of Panx1 channels was confirmed by using Panx1-deficient animals. Lacking Panx1 in neurons is sufficient to prevent the P2X7R-dependent upregulation of presynaptic strength; however, the P2X7R-dependent compensatory adjustment of synapse density requires both neuronal and glial Panx1. Together, our data supports an essential role for glial ATP signaling and Cx43HCs and Panx1 channels in the homeostatic adjustment of synaptic strength in hippocampal cultures upon chronic inactivity.





Similar content being viewed by others
References
Turrigiano, Nelson (2000) Hebb and homeostasis in neuronal plasticity. 10:358–364. https://doi.org/10.18438/eblip29427
Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135:422–435. https://doi.org/10.1016/j.cell.2008.10.008
Pozo K, Goda Y (2010) Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66:337–351. https://doi.org/10.1016/j.neuron.2010.04.028
Vitureira N, Goda Y (2013) The interplay between hebbian and homeostatic synaptic plasticity. J Cell Biol 203:175–186. https://doi.org/10.1083/jcb.201306030
Wierenga CJ, Walsh MF, Turrigiano GG (2006) Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol 96:2127–2133. https://doi.org/10.1152/jn.00107.2006
Perea G, Araque A (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317(5841):1083–1086. https://doi.org/10.1126/science.1144640
Perea G, Sur M, Araque A (2014) Neuron-glia networks: integral gear of brain function. Front Cell Neurosci 8:1–8. https://doi.org/10.3389/fncel.2014.00378
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFalpha. Science 295(5563):2282–2285. https://doi.org/10.1126/science.1067859
Stellwagen D, Malenka RC (2006) Synaptic scaling mediated by glial TNF-α. Nature 440:1054–1059. https://doi.org/10.1038/nature04671
Cunha RA, Ribeiro JA (2000) ATP as a presynaptic modulator. Life Sci 68:119–137. https://doi.org/10.1016/S0024-3205(00)00923-1
Butt AM (2011) ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol 22:205–213. https://doi.org/10.1016/j.semcdb.2011.02.023
Duster R, Prickaerts J, Blokland A (2013) Purinergic signaling and hippocampal long-term potentiation. Curr Neuropharmacol 12:37–43. https://doi.org/10.2174/1570159x113119990045
Evans RJ, Derkach V, Surprenant A (1992) ATP mediates fast synaptic transmission in mammalian neurons. Nature 357:503–505
Duan S, Neary J (2006) P2X7 receptors: properties and relevance to CNS function. Glia 54:738–746. https://doi.org/10.1002/glia
Sperlágh B, Vizi ES, Wirkner K, Illes P (2006) P2X7 receptors in the nervous system. Prog Neurobiol 78:327–346. https://doi.org/10.1016/j.pneurobio.2006.03.007
Sperlagh B, Kofalvi A, Deuchars J et al (2002) Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem 81:1196–1211
Cho J, Choi I, Jang I (2010) P2X7 receptors enhance glutamate release in hippocampal hilar neurons Neuroreport 21(13):865–70. https://doi.org/10.1097/WNR.0b013e32833d9142
Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A (2009) P2X receptors and synaptic plasticity. Neuroscience 158:137–148. https://doi.org/10.1016/j.neuroscience.2008.03.076
Khakh BS (2001) Molecular physiology of p2x receptors and atp signalling at synapses. Nat Rev Neurosci 2:165–174. https://doi.org/10.1038/35058521
Marcoli M, Cervetto C, Paluzzi P, Guarnieri S, Alloisio S, Thellung S, Nobile M, Maura G (2008) P2X7 pre-synaptic receptors in adult rat cerebrocortical nerve terminals: a role in ATP-induced glutamate release. J Neurochem 105:2330–2342. https://doi.org/10.1111/j.1471-4159.2008.05322.x
Abudara V, Retamal MA, Del Rio R, Orellana JA (2018) Synaptic functions of Hemichannels and Pannexons: a double-edged sword. Front Mol Neurosci 11:1–24. https://doi.org/10.3389/fnmol.2018.00435
Taruno A (2018) ATP release channels. Int J Mol Sci 19. https://doi.org/10.3390/ijms19030808
Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M (2008) Connexin 43 hemichannels are permeable to ATP. J Neurosci 28:4702–4711. https://doi.org/10.1523/jneurosci.5048-07.2008
Zhou KQ, Green CR, Bennet L, Gunn AJ, Davidson JO (2019) The role of connexin and pannexin channels in perinatal brain injury and inflammation. Front Physiol 10. https://doi.org/10.3389/fphys.2019.00141
Cheung G, Chever O, Rouach N (2014) Connexons and pannexons: newcomers in neurophysiology. Front Cell Neurosci 8:1–19
Ardiles AO, Toro-ayala G, Palacios AG et al (2014) Pannexin 1 regulates bidirectional hippocampal synaptic plasticity in adult mice. Front Cell Neurosci 8:326
Chever O, Pannasch U, Ezan P, Rouach N (2014) Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos Trans R Soc B 369:20130596
Wilson NR (2005) Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci 25:6221–6234. https://doi.org/10.1523/JNEUROSCI.3003-04.2005
de Jong APH, Schmitz SK, Toonen RFG, Verhage M (2012) Dendritic position is a major determinant of presynaptic strength. J Cell Biol 197:327–337. https://doi.org/10.1083/jcb.201112135
Herman MA, Ackermann F, Trimbuch T, Rosenmund C (2014) Vesicular glutamate transporter expression level affects synaptic vesicle release probability at hippocampal synapses in culture. J Neurosci 34:11781–11791. https://doi.org/10.1523/JNEUROSCI.1444-14.2014
Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, Lorenz RM, Kuyper CL et al (2011) Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 31:1461–1470. https://doi.org/10.1523/JNEUROSCI.3805-10.2011
Kay L, Humphreys L, Eickholt BJ, Burrone J (2011) Neuronal activity drives matching of pre- and postsynaptic function during synapse maturation. Nat Neurosci 14:688–690. https://doi.org/10.1038/nn.2826
Thiagarajan TC, Lindskog M, Tsien RW (2005) Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47:725–737. https://doi.org/10.1016/j.neuron.2005.06.037
Komoszynski M, Wojtczak A (1996) Apyrases (ATPdiphosphohydrolases, EC 3.6.1.5): function and relationship to ATPases. Biochim Biophys Acta 1310(2):233–241. https://doi.org/10.1016/0167-4889(95)00135-2
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067. https://doi.org/10.1152/physrev.00015.2002
Li M, Silberberg SD, Swartz KJ (2013) Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+. Proc Natl Acad Sci U S A 110:E3455–E3463. https://doi.org/10.1073/pnas.1308088110
Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP (2007) Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem 101:17–26. https://doi.org/10.1111/j.1471-4159.2006.04343.x
Hassel B, Paulsen RE, Johnsen A, Fonnum F (1992) Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res 576:120–124. https://doi.org/10.1016/0006-8993(92)90616-H
Shang XL, Wang QB, Liu XP, Yao XQ, Cao FY, Wang Q, Zhang JY, Wang JZ et al (2015) Fluorocitrate induced the alterations of memory-related proteins and tau hyperphosphorylation in SD rats. Neurosci Lett 584:230–235. https://doi.org/10.1016/j.neulet.2014.10.036
Fields RD, Stevens B (2000) ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 23:625–633. https://doi.org/10.1016/S0166-2236(00)01674-X
Wang Y, Haughey NJ, Mattson MP, Furukawa K (2004) Dual effects of ATP on rat hippocampal synaptic plasticity. Neuroreport 15:633–636. https://doi.org/10.1097/00001756-200403220-00012
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492. https://doi.org/10.1007/978-3-642-28863-0_5
Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TFC, Buckley NJ, Parson SH et al (2001) Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 21:7143–7152
Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, Carroll WA, Jarvis MF (2009) [3H]A-804598 ([3H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology 56:223–229. https://doi.org/10.1016/j.neuropharm.2008.06.012
Köles L, Fürst S, Illes P (2007) Purine ionotropic (P2X) receptors. Curr Pharm Des 13:2368–2384. https://doi.org/10.2174/138161207781368747
Samoilova M, Li J, Pelletier MR, Wentlandt K, Adamchik Y, Naus CC, Carlen PL (2003) Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: increased gap junctional function and expression. J Neurochem 86:687–699. https://doi.org/10.1046/j.1471-4159.2003.01893.x
Howard Evans W, Leybaert L (2007) Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes 14:265–273. https://doi.org/10.1080/15419060801891034
Wang N, De Bock M, Antoons G et al (2012) Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol 107:17. https://doi.org/10.1007/s00395-012-0304-2
Giaume C, Leybaert L, Naus CC, Sáez JC (2013) Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4 JUL:1–17. https://doi.org/10.3389/fphar.2013.00088
Abudara V, Bechberger J, Freitas-Andrade M et al (2014) The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci 8:1–8. https://doi.org/10.3389/fncel.2014.00306
Silverman W, Locovei S, Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol - Cell Physiol 295:C761–C767. https://doi.org/10.1152/ajpcell.00227.2008
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082. https://doi.org/10.1038/sj.emboj.7601378
Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D et al (2011) Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol 186:6553–6561. https://doi.org/10.4049/jimmunol.1100478
Boassa D, Nguyen P, Hu J, Ellisman MH, Sosinsky GE (2015) Pannexin2 oligomers localize in the membranes of endosomal vesicles in mammalian cells while pannexin1 channels traffic to the plasma membrane. Front Cell Neurosci 8:1–15. https://doi.org/10.3389/fncel.2014.00468
Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E (2012) ATP signaling is deficient in cultured pannexin1-null mouse astrocytes. Glia 60:1106–1116. https://doi.org/10.1002/glia.22338
Ma W, Hui H, Pelegrin P, Surprenant A (2009) Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328:409–418. https://doi.org/10.1124/jpet.108.146365
Locovei S, Bao L, Dahl G (2006) Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A 103:7655–7659. https://doi.org/10.1073/pnas.0601037103
Lindskog M, Li L, Groth RD, Poburko D, Thiagarajan TC, Han X, Tsien RW (2010) Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proc Natl Acad Sci U S A 107:21806–21811. https://doi.org/10.1073/pnas.1016399107
Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJL, Sutton MA (2010) Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron 68:1143–1158. https://doi.org/10.1016/j.neuron.2010.11.034
Orellana JA, Martinez AD, Retamal MA (2013) Gap junction channels and hemichannels in the CNS: Regulation by signaling molecules. Neuropharmacology 75:567–582. https://doi.org/10.1016/j.neuropharm.2013.02.020
Pannasch U, Vargová L, Reingruber J et al (2011) Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A 108:8467–8472. https://doi.org/10.1073/pnas.1016650108
Roux L, Madar A, Lacroix MM, Yi C, Benchenane K, Giaume C (2015) Astroglial connexin 43 hemichannels modulate olfactory bulb slow oscillations. J Neurosci 35:15339–15352. https://doi.org/10.1523/JNEUROSCI.0861-15.2015
Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci 100:13644–13649. https://doi.org/10.1073/pnas.2233464100
Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J et al (2011) Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A 108:20772–20777. https://doi.org/10.1073/pnas.1018262108
Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ (2008) N-WASP and the Arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem 283:15912–15920. https://doi.org/10.1074/jbc.M801555200
Wicki-Stordeur LE, Swayne LA (2013) Panx1 regulates neural stem and progenitor cell behaviours associated with cytoskeletal dynamics and interacts with multiple cytoskeletal elements. Cell Commun Signal 11:1. https://doi.org/10.1186/1478-811X-11-62
Sanchez-Arias JC, Liu M, Choi CSW, Ebert SN, Brown CE, Swayne LA (2019) Pannexin 1 regulates network ensembles and dendritic spine development in cortical neurons. eNeuro 6:ENEURO.0503–ENEU18.2019. https://doi.org/10.1523/ENEURO.0503-18.2019
Zorzi V, Paciello F, Ziraldo G, Peres C, Mazzarda F, Nardin C, Pasquini M, Chiani F et al (2017) Mouse Panx1 is dispensable for hearing acquisition and auditory function. Front Mol Neurosci 10:1–17. https://doi.org/10.3389/fnmol.2017.00379
Vitureira N, Letellier M, White IJ, Goda Y (2011) Differential control of presynaptic efficacy by postsynaptic N-cadherin and β-catenin. Nat Neurosci 15:81–89. https://doi.org/10.1038/nn.2995
Funding
The present study was supported by grants to N.V. and V.A. by the Comisión Sectorial de Investigación Científica (CSIC-UdelaR) and by the Program for the Development of Basic Sciences (PEDECIBA). A.R. and A.C received a Master Fellowship from the Agencia Nacional de Investigación e Innovación (ANII) and CSIC, UdelaR, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 935 kb)
Rights and permissions
About this article
Cite this article
Rafael, A., Cairus, A., Tizzoni, M. et al. Glial ATP and Large Pore Channels Modulate Synaptic Strength in Response to Chronic Inactivity. Mol Neurobiol 57, 2856–2869 (2020). https://doi.org/10.1007/s12035-020-01919-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01919-0