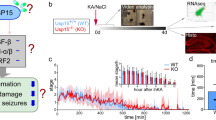
Abstract
Temporal lobe epilepsy (TLE) represents a devastating neurological condition, in which approximately 4/5 of patients remain refractory for anti-convulsive drugs. Epilepsy surgery biopsies often reveal the damage pattern of “hippocampal sclerosis” (HS) characterized not only by neuronal loss but also pronounced astrogliosis and inflammatory changes. Since TLE shares distinct pathogenetic aspects with multiple sclerosis (MS), we have here scrutinized therapeutic effects in experimental TLE of the immunmodulator fingolimod, which is established in MS therapy. Fingolimod targets sphingosine-phosphate receptors (S1PRs). mRNAs of fingolimod target S1PRs were augmented in two experimental post status epilepticus (SE) TLE mouse models (suprahippocampal kainate/pilocarpine). SE frequently induces chronic recurrent seizures after an extended latency referred to as epileptogenesis. Transient fingolimod treatment of mice during epileptogenesis after suprahippocampal kainate-induced SE revealed substantial reduction of chronic seizure activity despite lacking acute attenuation of SE itself. Intriguingly, fingolimod exerted robust anti-convulsive activity in kainate-induced SE mice treated in the chronic TLE stage and had neuroprotective and anti-gliotic effects and reduced cytotoxic T cell infiltrates. Finally, the expression profile of fingolimod target-S1PRs in human hippocampal biopsy tissue of pharmacoresistant TLE patients undergoing epilepsy surgery for seizure relief suggests repurposing of fingolimod as novel therapeutic perspective in focal epilepsies.







Similar content being viewed by others
References
Loscher W, Klitgaard H, Twyman RE, Schmidt D (2013) New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 12(10):757–776. https://doi.org/10.1038/nrd4126
Moshe SL, Perucca E, Ryvlin P, Tomson T (2015) Epilepsy: new advances. Lancet 385(9971):884–898. https://doi.org/10.1016/S0140-6736(14)60456-6
Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M (1998) Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51(5):1256–1262
Johnson AM, Sugo E, Barreto D, Hiew CC, Lawson JA, Connolly AM, Somerville E, Hasic E et al (2016) The severity of gliosis in hippocampal sclerosis correlates with pre-operative seizure burden and outcome after temporal lobectomy. Mol Neurobiol 53(8):5446–5456. https://doi.org/10.1007/s12035-015-9465-y
Steinhauser C, Grunnet M, Carmignoto G (2016) Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience 323:157–169. https://doi.org/10.1016/j.neuroscience.2014.12.047
Ryan K, Liang LP, Rivard C, Patel M (2014) Temporal and spatial increase of reactive nitrogen species in the kainate model of temporal lobe epilepsy. Neurobiol Dis 64:8–15. https://doi.org/10.1016/j.nbd.2013.12.006
Pitkanen A, Engel J Jr (2014) Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 11(2):231–241. https://doi.org/10.1007/s13311-014-0257-2
Kaminski RM, Rogawski MA, Klitgaard H (2014) The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurotherapeutics 11(2):385–400. https://doi.org/10.1007/s13311-014-0266-1
Tsai HC, Han MH (2016) Sphingosine-1-phosphate (S1P) and S1P signaling pathway: therapeutic targets in autoimmunity and inflammation. Drugs 76(11):1067–1079. https://doi.org/10.1007/s40265-016-0603-2
Paxinos G, Franklin, K (2012) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edition.
Bedner P, Dupper A, Huttmann K, Muller J, Herde MK, Dublin P, Deshpande T, Schramm J et al (2015) Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138(Pt 5):1208–1222. https://doi.org/10.1093/brain/awv067
Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S et al (2008) Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci 28(49):13341–13353. https://doi.org/10.1523/JNEUROSCI.1421-08.2008
Pitsch J, Schoch S, Gueler N, Flor PJ, van der Putten H, Becker AJ (2007) Functional role of mGluR1 and mGluR4 in pilocarpine-induced temporal lobe epilepsy. Neurobiol Dis 26(3):623–633
Pitsch J, Opitz T, Borm V, Woitecki A, Staniek M, Beck H, Becker AJ, Schoch S (2012) The presynaptic active zone protein RIM1alpha controls epileptogenesis following status epilepticus. J Neurosci 32(36):12384–12395. https://doi.org/10.1523/JNEUROSCI.0223-12.2012
Pitsch J, Becker AJ, Schoch S, Muller JA, de Curtis M, Gnatkovsky V (2017) Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus. Epilepsia 58:1159–1171. https://doi.org/10.1111/epi.13795
Gao F, Liu Y, Li X, Wang Y, Wei D, Jiang W (2012) Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav 103(2):187–196. https://doi.org/10.1016/j.pbb.2012.08.025
Leo A, Citraro R, Amodio N, De Sarro C, Gallo Cantafio ME, Constanti A, De Sarro G, Russo E (2017) Fingolimod exerts only temporary antiepileptogenic effects but longer-lasting positive effects on behavior in the WAG/Rij rat absence epilepsy model. Neurotherapeutics 14(4):1134–1147. https://doi.org/10.1007/s13311-017-0550-y
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3):659–661. https://doi.org/10.1096/fj.07-9574LSF
Blümcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ et al (2007) A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 113(3):235–244. https://doi.org/10.1007/s00401-006-0187-0
Kral T, Clusmann H, Urbach J, Schramm J, Elger CE, Kurthen M, Grunwald T (2002) Preoperative evaluation for epilepsy surgery (Bonn algorithm). Zentralbl Neurochir 63(3):106–110
van Loo KM, Schaub C, Pitsch J, Kulbida R, Opitz T, Ekstein D, Dalal A, Urbach H et al (2015) Zinc regulates a key transcriptional pathway for epileptogenesis via metal-regulatory transcription factor 1. Nat Commun 6:8688. https://doi.org/10.1038/ncomms9688
Pernhorst K, Herms S, Hoffmann P, Cichon S, Schulz H, Sander T, Schoch S, Becker AJ et al (2013) TLR4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure 22(8):675–678. https://doi.org/10.1016/j.seizure.2013.04.023
Jones RT, Barth AM, Ormiston LD, Mody I (2015) Evolution of temporal and spectral dynamics of pathologic high-frequency oscillations (pHFOs) during epileptogenesis. Epilepsia 56(12):1879–1889. https://doi.org/10.1111/epi.13218
Kunkel GT, Maceyka M, Milstien S, Spiegel S (2013) Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12(9):688–702. https://doi.org/10.1038/nrd4099
Gol M, Ghorbanian D, Hassanzadeh S, Javan M, Mirnajafi-Zadeh J, Ghasemi-Kasman M (2017) Fingolimod enhances myelin repair of hippocampus in pentylenetetrazol-induced kindling model. Eur J Pharm Sci 96:72–83. https://doi.org/10.1016/j.ejps.2016.09.016
Becker AJ (2017) Animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathol Appl Neurobiol 44:112–129. https://doi.org/10.1111/nan.12451
Blumcke I, Cross JH, Spreafico R (2013) The international consensus classification for hippocampal sclerosis: an important step towards accurate prognosis. Lancet Neurol 12(9):844–846. https://doi.org/10.1016/S1474-4422(13)70175-3
Aronica E, Crino PB (2011) Inflammation in epilepsy: clinical observations. Epilepsia 52(Suppl 3):26–32. https://doi.org/10.1111/j.1528-1167.2011.03033.x
Jeffery DR, Rammohan KW, Hawker K, Fox E (2016) Fingolimod: a review of its mode of action in the context of its efficacy and safety profile in relapsing forms of multiple sclerosis. Expert Rev Neurother 16(1):31–44. https://doi.org/10.1586/14737175.2016.1123094
Lee DH, Seubert S, Huhn K, Brecht L, Rotger C, Waschbisch A, Schlachetzki J, Klausmeyer A et al (2017) Fingolimod effects in neuroinflammation: regulation of astroglial glutamate transporters? PLoS One 12(3):e0171552. https://doi.org/10.1371/journal.pone.0171552
Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao CC, Wilz A et al (2017) Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A 114(8):2012–2017. https://doi.org/10.1073/pnas.1615413114
Das A, Arifuzzaman S, Kim SH, Lee YS, Jung KH, Chai YG (2017) FTY720 (fingolimod) regulates key target genes essential for inflammation in microglial cells as defined by high-resolution mRNA sequencing. Neuropharmacology 119:1–14. https://doi.org/10.1016/j.neuropharm.2017.03.034
Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA et al (2014) Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13(6):545–556. https://doi.org/10.1016/S1474-4422(14)70049-3
Acknowledgements
We thank Lioba Dammer and Vivian Ludwig for excellent technical assistance as well as Thoralf Opitz for critical discussion on the manuscript.
Funding
This work was supported by the Else-Kröner Fresenius Stiftung (JP, JCK, AJB), Novartis Pharma (to AJB), the Deutsche Forschungsgemeinschaft (SFB 1089 to AJB, SS, KMJ; FOR 2715 to AJB), the European Union’s Seventh Framework Program (FP7/2007-2013) under grant agreement n°602102 (EPITARGET; AJB, SS), Bundesministerium für Bildung und Forschung (01GQ0806, SS; the EraNet DeCipher to AJB), Fritz Thyssen Stiftung (Grant Ref 11.15.2.022MN) and the BONFOR program of the University of Bonn Medical Center (AJB, SS), EPICARE grant by Associazione Paolo Zorzi per le Neuroscienze and Ricerca Corrente, and RF151 grants of the Italian ministry of Health (MC).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Suppl. Fig. 1
S1PR subunit expression levels in experimental TLE. (A) mRNA expression levels of S1PRs in control animals in CA1 relative to the reference gene β-actin. S1PR2 and S1PR4 showed the lowest expression levels. S1PR5 and S1PR3 were expressed at moderate levels whereas S1PR1 had the highest expression level in CA1 region of naïve mice (n ≥ 5 per group). (B) No significant changes of S1PR4 (n ≥ 5 per group; 2way ANOVA treatment: p = 0.52) and (C) S1PR5 mRNA levels after pilocarpine-induced SE (n ≥ 5 per group; 2way ANOVA treatment: p = 0.88). Error bars indicate mean ± SEM. (PNG 100 kb)
Suppl. Fig. 2
S1PR expression levels depend on severity of hippocampal sclerosis. (A) The severity of hippocampal sclerosis significantly affects the mRNA expression level of S1PR1 in kainate-induced chronic TLE in CA1 (n ≥ 5; 2way ANOVA: ***p = 0.0002, F(2,58) = 9.921). S1PR1 is strongly increased in severe hippocampal characterized by a strong phenotype depicted in complete granule cell dispersion as well as a severe neuronal cell loss within the hippocampal sclerosis compared to the mild phenotype which resembles a moderate granule cell dispersion in the dentate gyrus and a slight neuronal cell loss in ipsilateral CA1 (Sidak’s post hoc: *p = 0.046). Also in the contralateral CA1 S1PR1 shows an abundance in the severe HS compared to sham-injected control CA1 (Sidak’s post hoc: **p = 0.0039) and mild sclerosis (**p = 0.0026). (B) The expression pattern of S1PR3 mRNA also depends on the severity of hippocampal sclerosis (2way ANOVA: ****p < 0.0001, F(2,58) = 19.61). S1PR3 mRNA shows a strong increased in severe hippocampal sclerosis (n = 5) compared to mild hippocampal sclerosis (n = 11) and sham-injected control animals (n = 11) in the ipsilateral (Sidak’s post hoc: ctrl vs. severe: ***p = 0.0002, mild vs severe: ***p = 0.0003) as well as in the contralateral side (Sidak’s post hoc: ctrl vs. severe: **p = 0.00233, mild vs severe: **p = 0.0021). Error bars indicate mean ± SEM. (PNG 72 kb)
Suppl. Fig. 3
No changes in gamma frequency during early acute SE. (A–D) EEG spectral analysis demonstrates no significant changes in gamma power increase (AUC, A, B) and duration of acute SE (C, D) between treated and untreated group in both hippocampal CA1. The following low wave delta EEG spectral pattern increase and duration is significantly increased in treated animals. This terminal phase of SE is not correlated with any seizure like behavior and the animal moved normally. (PNG 82 kb)
Suppl. Fig. 4
No changes in astrogliosis, activated microglia or neuronal cell loss in early treated experimental TLE. (A, B) No significant changes of reactive gliosis between untreated (n = 8) and treated group (n = 9). Mann-Whitney test: ipsilateral: p = 0.6, contralateral: p = 0.8. The staining was rated in relative extent of astrogliosis from 0 to 4 (0 = no reactive gliosis, 4 = strong gliosis). (C, D) No significant changes of activated microglia between untreated (n = 8) and treated group (n = 9). Mann-Whitney test: ipsilateral: p = 0.94, contralateral: p = 0.82. The staining was rated in relative extent of activated microglia from 0 to 4 (0 = no activated microglia, 4 = strong activated microglia) (E, F) No changes with respect to neuronal cell loss (n ≥ 8 per group). 2way ANOVA treatment: CA: p = 0.32; CA3: p = 0.16. Sidak’s post hoc: ipsilateral CA1: p = 0.28, contralateral CA1: 0.98; ipsilateral CA3: p = 0.68, contralateral CA3: p = 0.8. (PNG 138 kb)
Suppl. Fig. 5
Transient increase of S1PR3 mRNA in CA3 and DG in experimental TLE. (A–D) SE has also an impact on chronic mRNA expression levels of S1PR1 and 3 after pilocarpine-induced SE in CA3 similar to CA1 (n ≥ 5 for all groups; 2way ANOVA: S1PR1: *p = 0.026, F(1,33) = 5.45; S1PR3: ****p < 0.0001, F(1,33) = 127.8) and DG (2way ANOVA: S1PR1: ****p < 0.0001, F(1,33) = 78.65; S1PR3: ****p < 0.0001, F(1,33) = 127.2). (B) S1PR1 is significantly reduced 36 h after SE in CA3 (**p = 0.0073) whereas (C) S1PR3 shows an increase early after SE (12 h: ***p < 0.0001, 36 h: ****p < 0.0001, 72 h: ***p < 0.0001). (D) In DG S1PR1 is significantly reduced early after SE (12 h: ***p = 0.0002, 36 h: ****p < 0.0001, 72 h: **p = 0.008) and (E) S1PR3 is increased (12 h: ***p < 0.0001, 36 h: ****p < 0.0001, 72 h: ***p < 0.0001). Error bars indicate mean ± SEM. (PNG 115 kb)
Suppl. Fig. 6
S1PR3 protein expression levels 4 days after SE in CA1. Representative protein expression levels of S1PR3 in CA1 4 days after pilocarpine-induced SE with different co-stainings. (A) 4 days after SE S1PR3 expression shows an overlay with GFAP indicating a co-localization of S1PR3 and astrocytes (white arrows). (B) Co-staining with NeuN and (C) DAPI show an increased protein expression not exclusively in the neuronal nuclei but also in the somata (white arrowheads). (D) No co-localization is present with microglia visualized with co-staining using antibodies against Iba-1 (white arrow indicates increased expression on astrocytes). (PNG 5465 kb)
Suppl. Fig. 7
S1PR3 protein expression levels early after SE in CA1. (A) Time dependent representative protein expression levels of S1PR3 in CA1 after pilocarpine-induced SE. Strong abundance is present 4 days after. Overlay with GFAP shows a co-localization of S1PR3 in astrocytes (white arrows). Representative staining of S1PR3 in a control animal shows a sparse staining in the neurons of the control animal (upper row). Two days after SE the expression is higher in pyramidal neurons and expression is also present in the somata (white arrowheads). The strongest expression is present at four days after SE with a co-localisation on astrocytes and on neuronal somata. Seven days after SE the protein expression declines but signals are still present on astrocytes and neurons. (PNG 8839 kb)
Suppl. Fig. 8
S1PR3 protein expression levels early after SE in CA3. Representative protein expression levels of S1PR3 in CA3 after SE. The expression pattern is similar to CA1 (Fig. 6e). Strong protein increase of S1PR3 is present 4 days after SE. Co-localization analysis with GFAP reveals expression within the astrocytes (see arrows). (PNG 6974 kb)
Suppl. Fig. 9
S1PR3 protein expression levels early after SE in DG. Representative protein expression levels of S1PR3 in DG after SE. The expression pattern is similar to CA1 and CA3. Strongest increase of S1PR3 was present 4 days after SE. Co-localization with GFAP again reveals expression by astrocytes (see arrows). (PNG 7613 kb)
Suppl. Table 1
Sequences of primers that were used for real-time RT-PCR. (PNG 138 kb)
Suppl. Table 2
Antibodies and dilutions for protein Expression. (PNG 203 kb)
Rights and permissions
About this article
Cite this article
Pitsch, J., Kuehn, J.C., Gnatkovsky, V. et al. Anti-epileptogenic and Anti-convulsive Effects of Fingolimod in Experimental Temporal Lobe Epilepsy. Mol Neurobiol 56, 1825–1840 (2019). https://doi.org/10.1007/s12035-018-1181-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1181-y