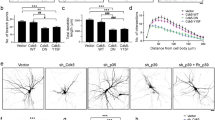
Abstract
Proper formation of neuronal dendritic branching is crucial for correct brain function. The number and distribution of receptive synaptic contacts are defined by the size and shape of dendritic arbors. Our previous research found that protocadherin 11 X-linked protein (Pcdh11x) is predominantly expressed in neurons and has an influence on dendritic branching. In this study, gain-of-function and loss-of-function experiments revealed that Pcdh11x acts as a negative regulator of dendritic branching in cultured cortical neurons derived from embryonic day 16 mice. Overexpression of wild-type Pcdh11x (Pcdh11x-GFP) reduced dendritic complexity, whereas knockdown of Pcdh11x increased dendritic branching. It was further demonstrated that Pcdh11x activates PI3K/AKT signaling to negatively regulate dendritic branching.




Similar content being viewed by others
References
Branco T, Clark BA, Hausser M (2010) Dendritic discrimination of temporal input sequences in cortical neurons. Science 329:1671–5
Branco T, Hausser M (2011) Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 69:885–92
Ghysen A (2003) Dendritic arbors: a tale of living tiles. Curr Biol 13:R427–9
Gidon A, Segev I (2012) Principles governing the operation of synaptic inhibition in dendrites. Neuron 75:330–41
Hattori D, Millard SS, Wojtowicz WM, Zipursky SL (2008) Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol 24:597–620
Hausser M, Spruston N, Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science 290:739–44
Jan YN, Jan LY (2010) Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11:316–28
Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M (2005) Control of dendritic arborization by the phosphoinositide-3'-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci 25:11300–12
Kutzing MK, Langhammer CG, Luo V, Lakdawala H, Firestein BL (2010) Automated Sholl analysis of digitized neuronal morphology at multiple scales. J Vis Exp 45:pii:2354. doi:10.3791/2354
Langhammer CG, Previtera ML, Sweet ES, Sran SS, Chen M, Firestein BL (2010) Automated Sholl analysis of digitized neuronal morphology at multiple scales: whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A 77:1160–8
Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J (2012) Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature 490:397–401
Leemhuis J, Boutillier S, Barth H et al (2004) Rho GTPases and phosphoinositide 3-kinase organize formation of branched dendrites. J Biol Chem 279:585–96
Lin YC, Koleske AJ (2010) Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci 33:349–78
Morishita H, Yagi T (2007) Protocadherin family: diversity, structure, and function. Curr Opin Cell Biol 19:584–92
Parrish JZ, Emoto K, Kim MD, Jan YN (2007) Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 30:399–423
Sholl D (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387–406
Sperry R (1963) Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A 50:703–10
Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K (1988) Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature 333:177–80
Suo L, Lu H, Ying G, Capecchi MR, Wu Q (2012) Protocadherin clusters and cell adhesion kinase regulate dendrite complexity through Rho GTPase. J Mol Cell Biol 4:362–76
Takeichi M (2007) The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci 8:11–20
Toyoda S, Kawaguchi M, Kobayashi T et al (2014) Developmental epigenetic modification regulates stochastic expression of clustered protocadherin genes, generating single neuron diversity. Neuron 82:94–108
Yagi T (2013) Genetic basis of neuronal individuality in the mammalian brain. J Neurogenet 27:97–105
Ye B, Jan YN (2005) The cadherin superfamily and dendrite development. Trends Cell Biol 15:64–7
Yu X, Malenka RC (2003) Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci 6:1169–77
Zhang P, Wu C, Liu N et al (2014) Protocadherin 11 X regulates differentiation and proliferation of neural stem cell in vitro and in vivo. J Mol Neurosci 54(2):199–210
Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V (1998) AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143:1295–304
Zipursky SL, Sanes JR (2010) Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell 143:343–53
Acknowledgments
This study was funded by Military Medical Project (BWS11J002, BWS12J010) and National Natural Science Foundation (No. 81401031). We thank Springer Language Editing (Project No.: C1501-40452-WuCuiying) for assisting in the preparation of this manuscript. The authors would like to thank all the anonymous reviewers for their valuable comments on how to improve the quality of this paper.
Conflict of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, C., Niu, L., Yan, Z. et al. Pcdh11x Negatively Regulates Dendritic Branching. J Mol Neurosci 56, 822–828 (2015). https://doi.org/10.1007/s12031-015-0515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0515-8