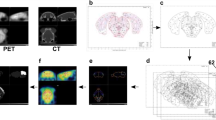
Abstract
Purpose
Plastic changes in the central auditory system involving the GABAergic system accompany age-related hearing loss. Such processes can be investigated with positron emission tomography (PET) imaging using [18F]flumazenil ([18F]FMZ). Here, [18F]FMZ PET-based modeling approaches allow a simple and reliable quantification of GABAA receptor binding capacity revealing regional differences and age-related changes.
Procedures
Sixty-minute list-mode PET acquisitions were performed in 9 young (range 5–6 months) and 11 old (range 39–42 months) gerbils, starting simultaneously with the injection of [18F]FMZ via femoral vein. Non-displaceable binding potentials (BPnd) with pons as reference region were calculated for auditory cortex (AC), inferior colliculus (IC), medial geniculate body (MGB), somatosensory cortex (SC), and cerebellum (CB) using (i) a two-tissue compartment model (2TCM), (ii) the Logan plot with image-derived blood-input (Logan (BI)), (iii) a simplified reference tissue model (SRTM), and (iv) the Logan reference model (Logan (RT)). Statistical parametric mapping analysis (SPM) comparing young and old gerbils was performed using 3D parametric images for BPnd based on SRTM. Results were verified with in vitro autoradiography from five additional young gerbils. Model assessment included the Akaike information criterion (AIC). Hearing was evaluated using auditory brainstem responses.
Results
BPnd differed significantly between models (p < 0.0005), showing the smallest mean difference between 2TCM as reference and SRTM as simplified procedure. SRTM revealed the lowest AIC values. Both volume of distribution (r2 = 0.8793, p = 0.018) and BPnd (r2 = 0.8216, p = 0.034) correlated with in vitro autoradiography data. A significant age-related decrease of receptor binding was observed in auditory (AC, IC, MGB) and other brain regions (SC and CB) (p < 0.0001, unpaired t test) being confirmed by SPM using pons as reference (p < 0.0001, uncorrected).
Conclusion
Imaging of GABAA receptor binding capacity in gerbils using [18F]FMZ PET revealed SRTM as a simple and robust quantification method of GABAA receptors. Comparison of BPnd in young and old gerbils demonstrated an age-related decrease of GABAA receptor binding.




Similar content being viewed by others
References
Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A 97:9226–9233
Cherry SR, Gambhir SS (2001) Use of positron emission tomography in animal research. ILAR J 42:219–232
Ametamey SM, Honer M, Schubiger PA (2008) Molecular imaging with PET. Chem Rev 108:1501–1516
Marriott CJ, Cadorette JE, Lecomte R, Scasnar V, Rousseau J, van Lier J (1994) High-resolution PET imaging and quantitation of pharmaceutical biodistributions in a small animal using avalanche photodiode detectors. J Nucl Med 35:1390–1396
Farde L (1996) The advantage of using positron emission tomography in drug research. Trends Neurosci 19:211–214
Fujita M (2001) In vivo receptor imaging with PET and SPET-pitfalls in quantification. Int Rev Psychiatry 13:34–39
Dupont P, Warwick J (2009) Kinetic modelling in small animal imaging with PET. Methods 48:98–103
Ingvar M, Eriksson L, Rogers GA, Stone-Elander S, Widén L (1991) Rapid feasibility studies of tracers for positron emission tomography: high-resolution PET in small animals with kinetic analysis. J Cereb Blood Flow Metab 11:926–931
Maggi A, Schmidt MJ, Ghetti B, Enna SJ (1979) Effect of aging on neurotransmitter receptor binding in rat and human brain. Life Sci 24:367–373
McQuail JA, Frazier CJ, Bizon JL (2015) Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med 21:450–460
Owens DF, Kriegstein AR (2002) Is there more to gaba than synaptic inhibition? Nat Rev Neurosci 3:715–727
Caspary DM, Llano DA (2018) Aging processes in the subcortical auditory system. Oxford University Press, Oxford. https://doi.org/10.1093/oxfordhb/9780190849061.013.16
Nutt D (2006) GABAA receptors: subtypes, regional distribution, and function. J Clin Sleep Med 2:S7–S11
Young AB, Chu D (1990) Distribution of GABAA and GABAB receptors in mammalian brain: potential targets for drug development. Drug Dev Res 21:161–167
Bowery NG, Hudson AL, Price GW (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383
Nutt DJ, Malizia AL (2001) New insights into the role of the GABAA;—benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 179:390–396
Schultz LM (1997) REVIEW: GABAergic inhibitory processes and hippocampal long-term potentiation. Neuroscientist 3:226–236
Rodnick ME, Hockley BG, Sherman P, Quesada C, Battle MR, Jackson A, Linder KE, Macholl S, Trigg WJ, Kilbourn MR, Scott PJH (2013) Novel fluorine-18 PET radiotracers based on flumazenil for GABAA imaging in the brain. Nucl Med Biol 40:901–905
Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABA(a) receptors. J Biol Chem 287:40224–40231
Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK (1999) Age-related changes in GABAA receptor subunit composition and function in rat auditory system. Neuroscience 93:307–312
Mandema JW, Gubbens-Stibbe JM, Danhof M (1991) Stability and pharmacokinetics of flumazenil in the rat. Psychopharmacology 103:384–387
Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen AJ, Krasikova RN, Farde L (2009) [18F]flumazenil binding to central benzodiazepine receptor studies by PET--quantitative analysis and comparisons with [11C]flumazenil. NeuroImage 45:891–902
Caspary DM, Ling L, Turner JG, Hughes LF (2008) Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211:1781–1791
Caspary DM, Milbrandt JC, Helfert RH (1995) Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol 30:349–360
Milbrandt JC, Albin RL, Caspary DM (1994) Age-related decrease in GABAB receptor binding in the Fischer 344 rat i inferior colliculus. Neurobiol Aging 15:699–703
Milbrandt JC, Albin RL, Turgeon SM, Caspary DM (1996) GABAA receptor binding in the aging rat inferior colliculus. Neuroscience 73:449–458
Ryan A (1976) Hearing sensitivity of the mongolian gerbil, Meriones unguiculatis. J Acoust Soc Am 59:1222–1226
Mills JH, Schmiedt RA, Kulish LF (1990) Age-related changes in auditory potentials of mongolian gerbil. Hear Res 46:201–210
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Liang H, Zou G (2008) Improved AIC selection strategy for survival analysis. Comput Stat Data Anal 52:2538–2548
Preshlock S, Calderwood S, Verhoog S, Tredwell M, Huiban M, Hienzsch A, Gruber S, Wilson TC, Taylor NJ, Cailly T, Schedler M, Collier TL, Passchier J, Smits R, Mollitor J, Hoepping A, Mueller M, Genicot C, Mercier J, Gouverneur V (2016) Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem Commun (Camb) 52:8361–8364
Pachitariu M, Lyamzin DR, Sahani M, Lesica NA (2015) State-dependent population coding in primary auditory cortex. J Neurosci 35:2058–2073
Beutelmann R, Laumen G, Tollin D, Klump GM (2015) Amplitude and phase equalization of stimuli for click evoked auditory brainstem responses. J Acoust Soc Am 137:EL71–EL77
Laumen G, Tollin DJ, Beutelmann R, Klump GM (2016) Aging effects on the binaural interaction component of the auditory brainstem response in the Mongolian gerbil: effects of interaural time and level differences. Hear Res 337:46–58
Kessler M, Mamach M, Beutelmann R, Bankstahl JP, Bengel FM, Klump GM, Berding G (2018) Activation in the auditory pathway of the gerbil studied with 18F-FDG PET: effects of anesthesia. Brain Struct Funct 223:4293–4305
Radtke-Schuller S, Schuller G, Angenstein F, Grosser OS, Goldschmidt J, Budinger E (2016) Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct Funct 221(Suppl 1):1–272
Thackeray JT, Bankstahl JP, Bengel FM (2015) Impact of image-derived input function and fit time intervals on Patlak quantification of myocardial glucose uptake in mice. J Nucl Med 56:1615–1621
Kuntner C (2014) Kinetic modeling in pre-clinical positron emission tomography. Z Med Phys 24:274–285
Koeppe RA, Holthoff VA, Frey KA, Kilbourn MR, Kuhl DE (1991) Compartmental analysis of [11C]flumazenil kinetics for the estimation of ligand transport rate and receptor distribution using positron emission tomography. J Cereb Blood Flow Metab 11:735–744
Dedeurwaerdere S, Gregoire M-C, Vivash L, Roselt P, Binns D, Fookes C, Greguric I, Pham T, Loc’h C, Katsifis A, Hicks RJ, O’Brien TJ, Myers DE (2009) In-vivo imaging characteristics of two fluorinated flumazenil radiotracers in the rat. Eur J Nucl Med Mol Imaging 36:958–965
Vivash L, Gregoire MC, Lau EW, Ware RE, Binns D, Roselt P, Bouilleret V, Myers DE, Cook MJ, Hicks RJ, O'Brien TJ (2013) 18F-flumazenil: a gamma-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med 54:1270–1277
Morris JS, Friston KJ, Dolan RJ (1998) Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc R Soc B Biol Sci 265:649–657
Gunn RN, Gunn SR, Turkheimer FE, Aston JAD, Cunningham VJ (2002) Positron emission tomography compartmental models: a basis pursuit strategy for kinetic modeling. J Cereb Blood Flow Metab 22:1425–1439
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. NeuroImage 4:153–158
Klumpers U, H M, Veltman DJ, Boellaard R et al (2007) Comparison of plasma input and reference tissue models for analysing [11C]flumazenil studies. J Cereb Blood Flow Metab 28:579–587
Geeraerts T, P Coles J, I Aigbirhio F et al (2011) Validation of reference tissue modelling for [11C]flumazenil positron emission tomography following head injury. Ann Nucl Med 25:396–405
Miederer I, Ziegler SI, Liedtke C, Spilker ME, Miederer M, Sprenger T, Wagner KJ, Drzezga A, Boecker H (2009) Kinetic modelling of [11C]flumazenil using data-driven methods. Eur J Nucl Med Mol Imaging 36:659–670
Lopes Alves I, Vállez García DV, Parente A, Doorduin J, da Silva AMM, Koole M, Dierckx R, Willemsen A, Boellaard R (2018) Parametric imaging of [11C]flumazenil binding in the rat brain. Mol Imaging Biol 20:114–123
Zanotti-Fregonara P, Chen K, Liow J-S, Fujita M, Innis RB (2011) Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab 31:1986–1998
Hammers A, Panagoda P, Heckemann RA et al (2007) [11C]flumazenil PET in temporal lobe epilepsy: do we need an arterial input function or kinetic modeling? J Cereb Blood Flow Metab 28:207–216
Lanz B, Poitry-Yamate C, Gruetter R (2014) Image-derived input function from the vena cava for 18F-FDG PET studies in rats and mice. J Nucl Med 55:1380–1388
Tsartsalis S, Tournier BB, Graf CE, Ginovart N, Ibáñez V, Millet P (2018) Dynamic image denoising for voxel-wise quantification with statistical parametric mapping in molecular neuroimaging. PLoS One 13:e0203589–e0203589
Golla SSV, Adriaanse SM, Yaqub M, Windhorst AD, Lammertsma AA, van Berckel BNM, Boellaard R (2017) Model selection criteria for dynamic brain PET studies. EJNMMI Physics 4:30
Parente A, Vállez García D, Shoji A, Lopes Alves I, Maas B, Zijlma R, Dierckx RAJO, Buchpiguel CA, de Vries EFJ, Doorduin J (2017) Contribution of neuroinflammation to changes in [11C]flumazenil binding in the rat brain: evaluation of the inflamed pons as reference tissue. Nucl Med Biol 49:50–56
Barnard EA, Skolnick P, Olsen RW et al (1998) International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291–313
Palner M, Beinat C, Banister S, Zanderigo F, Park JH, Shen B, Hjoernevik T, Jung JH, Lee BC, Kim SE, Fung L, Chin FT (2016) Effects of common anesthetic agents on [18F]flumazenil binding to the GABAA receptor. EJNMMI Res 6:80
Gyulai MD, Ferenc E, Mintun MD et al (2001) Dose-dependent enhancement of in vivo GABAA–benzodiazepine receptor binding by isoflurane. Anesthesiology 95:585–593
Rissman RA, De Blas AL, Armstrong DM (2007) GABAA receptors in aging and Alzheimer’s disease. J Neurochem 103:1285–1292
Rissman RA, Mobley WC (2011) Implications for treatment: GABAA receptors in aging, down syndrome and Alzheimer’s disease. J Neurochem 117:613–622
Prieto E, Collantes M, Delgado M, Juri C, García-García L, Molinet F, Fernández-Valle ME, Pozo MA, Gago B, Martí-Climent JM, Obeso JA, Peñuelas I (2011) Statistical parametric maps of 18F-FDG PET and 3-D autoradiography in the rat brain: a cross-validation study. Eur J Nucl Med Mol Imaging 38:2228–2237
Benfenati F, Cimino M, Agnati LF, Fuxe K (1986) Quantitative autoradiography of central neurotransmitter receptors: methodological and statistical aspects with special reference to computer-assisted image analysis. Acta Physiol Scand 128:129–146
Pike VW, Halldin C, Crouzel C, Barré L, Nutt DJ, Osman S, Shah F, Turton DR, Waters SL (1993) Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites--current status. Nucl Med Biol 20:503–525
Andersson JD, Halldin C (2013) PET radioligands targeting the brain GABAA /benzodiazepine receptor complex. J Label Compd Radiopharm 56:196–206
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000) GABA(a) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101:815–850
Lee K-Y (2013) Pathophysiology of age-related hearing loss (peripheral and central). Korean J Audiol 17:45–49
Banay-Schwartz M, Palkovits M, Lajtha A (1993) Heterogeneous distribution of functionally important amino acids in brain areas of adult and aging humans. Neurochem Res 18:417–423
Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arneric SP (1990) Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci 10:2363–2372
Gutierrez A, Khan ZU, Morris SJ, De Blas AL (1994) Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci 14:7469–7477
Milbrandt JC, Hunter C, Caspary DM (1997) Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol 379:455–465
Godfrey DA, Chen K, O'Toole TR, Mustapha A (2017) Amino acid and acetylcholine chemistry in the central auditory system of young, middle-aged and old rats. Hear Res 350:173–188
Burianova J, Ouda L, Profant O, Syka J (2009) Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44:161–169
Ymer S, Draguhn A, Wisden W, Werner P, Keinänen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH (1990) Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J 9:3261–3267
Hoekzema E, Rojas S, Herance R, Pareto D, Abad S, Jiménez X, Figueiras FP, Popota F, Ruiz A, Flotats N, Fernández FJ, Rocha M, Rovira M, Víctor VM, Gispert JD (2012) In vivo molecular imaging of the GABA/benzodiazepine receptor complex in the aged rat brain. Neurobiol Aging 33:1457–1465
Hamezah HS, Durani LW, Ibrahim NF, Yanagisawa D, Kato T, Shiino A, Tanaka S, Damanhuri HA, Ngah WZW, Tooyama I (2017) Volumetric changes in the aging rat brain and its impact on cognitive and locomotor functions. Exp Gerontol 99:69–79
Acknowledgements
We wish to thank Alexander Kanwischer and Petra Felsch for their support in performing the PET scans.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC 2177/1 - Project ID 390895286.
Conflict of InterestThe authors declare that they have no conflict of interest.
Ethical ApprovalProcedures, care, and treatment of the gerbils were conducted in conformity with the institutional responsible person for animal welfare at Hannover Medical School and in accordance with a permit issued by the responsible local authority (Landesamt für Verbraucherschutz und Lebensmittelsicherheit, LAVES, Lower Saxony, Germany). The use of animals in this study also complies with the “Guide for the Care and Use of Laboratory Animals” (NIH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 96 kb)
Rights and permissions
About this article
Cite this article
Kessler, M., Mamach, M., Beutelmann, R. et al. GABAA Receptors in the Mongolian Gerbil: a PET Study Using [18F]Flumazenil to Determine Receptor Binding in Young and Old Animals. Mol Imaging Biol 22, 335–347 (2020). https://doi.org/10.1007/s11307-019-01371-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01371-0