Abstract
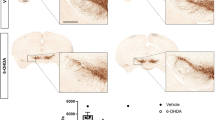
Arachidonic acid (AA) signaling is upregulated in the caudate-putamen and frontal cortex of unilaterally 6-hydroxydopamine (6-OHDA) lesioned rats, a model for asymmetrical Parkinson disease. AA signaling can be coupled to D2-like receptor initiated AA hydrolysis from phospholipids by cytosolic phospholipase A2 (cPLA2) and subsequent metabolism by cyclooxygenase (COX)-2. In unilaterally 6-OHDA- and sham-lesioned rats, we measured brain expression of cPLA2, other PLA2 enzymes, and COX-2. Activity and protein levels of cPLA2 were significantly higher as was COX-2-protein in caudate-putamen, frontal cortex and remaining brain on the lesioned compared to intact side of the 6-OHDA lesioned rats, and compared to sham brain. Secretory sPLA2 and Ca2+-independent iPLA2 expression did not differ between sides or groups. Thus, the tonically increased ipsilateral AA signal in the lesioned rat corresponds to upregulated cPLA2 and COX-2 expression within the AA metabolic cascade, which may contribute to symptoms and pathology in Parkinson disease.
Similar content being viewed by others
Abbreviations
- AA:
-
Arachidonic acid
- COX-2:
-
Cyclooxygenase-2
- cPLA2 :
-
Cytosolic phospholipase A2
- DAT:
-
Dopamine reuptake transporter
- iPLA2 :
-
Calcium independent PLA2
- NMDA:
-
N-methyl-d-aspartate
- sPLA2 :
-
Secretory PLA2
- 5-HT:
-
5-hydroxytryptamine (serotonin)
- 6-OHDA:
-
6-hydroxydopamine
References
Hornykiewicz O (1982) Imbalance of brain monoamines and clinical disorders. Prog Brain Res 55:419–429
Heiss WD, Hilker R (2004) The sensitivity of 18-fluorodopa positron emission tomography and magnetic resonance imaging in Parkinson’s disease. Eur J Neurol 11:5–12
Lang AE, Lozano AM (1998) Parkinson’s disease. Second of two parts. N Engl J Med 339:1130–1143
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Ungerstedt U (1971) Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 367:69–93
Yuan H, Sarre S, Ebinger G, Michotte Y (2005) Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods 144:35–45
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317
Jeon BS, Jackson-Lewis V, Burke RE (1995) 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration 4:131–137
Arnt J, Hyttel J (1985) Differential involvement of dopamine D-1 and D-2 receptors in the circling behaviour induced by apomorphine, SK & F 38393, pergolide and LY 171555 in 6-hydroxydopamine-lesioned rats. Psychopharmacology (Berl) 85:346–352
Cadet JL, Zhu SM (1992) The intrastriatal 6-hydroxydopamine model of hemiparkinsonism: quantitative receptor autoradiographic evidence of correlation between circling behavior and presynaptic as well as postsynaptic nigrostriatal markers in the rat. Brain Res 595:316–326
Zuch CL, Nordstroem VK, Briedrick LA, Hoernig GR, Granholm AC, Bickford PC (2000) Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J Comp Neurol 427:440–454
Nikolaus S, Larisch R, Beu M, Forutan F, Vosberg H, Muller-Gartner HW (2003) Bilateral increase in striatal dopamine D2 receptor density in the 6-hydroxydopamine-lesioned rat: a serial in vivo investigation with small animal PET. Eur J Nucl Med Mol Imaging 30:390–395
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr et al (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Graham WC, Crossman AR, Woodruff GN (1990) Autoradiographic studies in animal models of hemi-parkinsonism reveal dopamine D2 but not D1 receptor supersensitivity. I. 6-OHDA lesions of ascending mesencephalic dopaminergic pathways in the rat. Brain Res 514:93–102
Chalon S, Emond P, Bodard S, Vilar MP, Thiercelin C, Besnard JC et al (1999) Time course of changes in striatal dopamine transporters and D2 receptors with specific iodinated markers in a rat model of Parkinson’s disease. Synapse 31:134–139
Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY et al (1991) A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65:1043–1051
Axelrod J (1990) Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans 18:503–507
Nilsson CL, Hellstrand M, Ekman A, Eriksson E (1998) Direct dopamine D2-receptor-mediated modulation of arachidonic acid release in transfected CHO cells without the concomitant administration of a Ca2+-mobilizing agent. Br J Pharmacol 124:1651–1658
Vial D, Piomelli D (1995) Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonate-specific phospholipase A2. J Neurochem 64:2765–2772
Ong WY, Sandhya TL, Horrocks LA, Farooqui AA (1999) Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J Hirnforsch 39:391–400
Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P (1996) COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA 93:2317–2321
Hayakawa T, Chang MC, Bell JM, Seeman R, Rapoport SI, Appel NM (1998) Fatty acid incorporation depicts brain activity in a rat model of Parkinson’s disease. Brain Res 807:177–181
Bhattacharjee AK, Meister LM, Chang L, Bazinet RP, White L, Rapoport SI (2007) In vivo imaging of disturbed pre- and post-synaptic dopaminergic signaling via arachidonic acid in a rat model of Parkinson’s disease. Neuroimage 37:1112–1121
Hayakawa T, Chang MC, Rapoport SI, Appel NM (2001) Selective dopamine receptor stimulation differentially affects [3H]arachidonic acid incorporation, a surrogate marker for phospholipase A2-mediated neurotransmitter signal transduction, in a rodent model of Parkinson’s disease. J Pharmacol Exp Ther 296:1074–1084
Shimizu T, Wolfe LS (1990) Arachidonic acid cascade and signal transduction. J Neurochem 55:1–15
Fitzpatrick FA, Soberman R (2001) Regulated formation of eicosanoids. J Clin Invest 107:1347–1351
Basselin M, Chang L, Bell JM, Rapoport SI (2005) Chronic lithium chloride administration to unanesthetized rats attenuates brain dopamine D2-like receptor-initiated signaling via arachidonic acid. Neuropsychopharmacology 30:1064–1075
Rapoport SI (2008) Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids 79:153–156
Dennis EA (1994) Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 269:13057–13060
Basselin M, Villacreses NE, Langenbach R, Ma K, Bell JM, Rapoport SI (2006) Resting and arecoline-stimulated brain metabolism and signaling involving arachidonic acid are altered in the cyclooxygenase-2 knockout mouse. J Neurochem 96:669–679
Takano T, Panesar M, Papillon J, Cybulsky AV (2000) Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat 60:15–26
Strokin M, Sergeeva M, Reiser G (2007) Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. J Neurochem 102:1771–1782
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493
Inaji M, Okauchi T, Ando K, Maeda J, Nagai Y, Yoshizaki T et al (2005) Correlation between quantitative imaging and behavior in unilaterally 6-OHDA-lesioned rats. Brain Res 1064:136–145
Sauer H, Oertel WH (1994) Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59:401–415
Lucas KK, Dennis EA (2005) Distinguishing phospholipase A2 types in biological samples by employing group-specific assays in the presence of inhibitors. Prostaglandins Other Lipid Mediat 77:235–248
Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA (1999) Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal Biochem 269:278–288
Bhattacharjee AK, Chang L, Lee HJ, Bazinet RP, Seemann R, Rapoport SI (2005) D2 but not D1 dopamine receptor stimulation augments brain signaling involving arachidonic acid in unanesthetized rats. Psychopharmacology (Berl) 180:735–742
Bhattacharjee AK, Chang L, White L, Bazinet RP, Rapoport SI (2006) D-Amphetamine stimulates D2 dopamine receptor-mediated brain signaling involving arachidonic acid in unanesthetized rats. J Cereb Blood Flow Metab 26:1378–1388
Kanterman RY, Axelrod J, Felder CC (1990) Alpha-1 adrenergic receptor mediates the release of arachidonic acid in spinal cord neurons independent of phosphatidylinositol-specific phospholipase C. Adv Second Messenger Phosphoprotein Res 24:158–163
Schinelli S, Paolillo M, Corona GL (1994) Opposing actions of D1- and D2-dopamine receptors on arachidonic acid release and cyclic AMP production in striatal neurons. J Neurochem 62:944–949
Wei S, Ong WY, Thwin MM, Fong CW, Farooqui AA, Gopalakrishnakone P et al (2003) Group IIA secretory phospholipase A2 stimulates exocytosis and neurotransmitter release in pheochromocytoma-12 cells and cultured rat hippocampal neurons. Neuroscience 121:891–898
Ribeiro MJ, Vidailhet M, Loc’h C, Dupel C, Nguyen JP, Ponchant M et al (2002) Dopaminergic function and dopamine transporter binding assessed with positron emission tomography in Parkinson disease. Arch Neurol 59:580–586
Ichise M, Kim YJ, Ballinger JR, Vines D, Erami SS, Tanaka F et al (1999) SPECT imaging of pre- and postsynaptic dopaminergic alterations in L-dopa-untreated PD. Neurology 52:1206–1214
Ross BM, Mamalias N, Moszczynska A, Rajput AH, Kish SJ (2001) Elevated activity of phospholipid biosynthetic enzymes in substantia nigra of patients with Parkinson’s disease. Neuroscience 102:899–904
Giovacchini G, Lerner A, Toczek MT, Fraser C, Ma K, DeMar JC et al (2004) Brain incorporation of 11C-arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med 45:1471–1479
Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM (1995) Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 378:180–182
Esposito G, Giovacchini G, Der M, Liow JS, Bhattacharjee AK, Ma K et al (2007) Imaging signal transduction via arachidonic acid in the human brain during visual stimulation, by means of positron emission tomography. Neuroimage 34:1342–1351
Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M et al (2008) Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J Nucl Med 49:1414–1421
Tay A, Simon JS, Squire J, Hamel K, Jacob HJ, Skorecki K (1995) Cytosolic phospholipase A2 gene in human and rat: chromosomal localization and polymorphic markers. Genomics 26:138–141
Pardue S, Rapoport SI, Bosetti F (2003) Co-localization of cytosolic phospholipase A2 and cyclooxygenase-2 in Rhesus monkey cerebellum. Brain Res Mol Brain Res 116:106–114
Balsinde J, Balboa MA, Dennis EA (1998) Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc Natl Acad Sci USA 95:7951–7956
Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O (2002) Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci 15:991–998
Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN et al (2004) Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem 88:1168–1178
Richardson RL, Kim EM, Gardiner T, O’Hare E (2005) Chronic intracerebroventricular infusion of lipopolysaccharide: effects of ibuprofen treatment and behavioural and histopathological correlates. Behavioural pharmacology 16:531–541
Lee H, Villacreses NE, Rapoport SI, Rosenberger TA (2004) In vivo imaging detects a transient increase in brain arachidonic acid metabolism: a potential marker of neuroinflammation. J Neurochem 91:936–945
Anthonsen MW, Solhaug A, Johansen B (2001) Functional coupling between secretory and cytosolic phospholipase A2 modulates tumor necrosis factor-alpha- and interleukin-1beta-induced NF-kappa B activation. J Biol Chem 276:30527–30536
Mirza B, Hadberg H, Thomsen P, Moos T (2000) The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 95:425–432
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Mov Disord 23:474–483
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397
Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202:17–20
Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ et al (2005) Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol 58:963–967
Wahner AD, Bronstein JM, Bordelon YM, Ritz B (2007) Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 69:1836–1842
Bornebroek M, de Lau LM, Haag MD, Koudstaal PJ, Hofman A, Stricker BH et al (2007) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology 28:193–196
Samii A, Etminan M, Wiens MO, Jafari S (2009) NSAID use and the risk of Parkinson’s disease: systematic review and meta-analysis of observational studies. Drugs Aging 26:769–779
Xiao L, Patterson PS, Yang C, Lal AA (1999) Role of eicosanoids in the pathogenesis of murine cerebral malaria. Am J Trop Med Hyg 60:668–673
McGahon B, Clements MP, Lynch MA (1997) The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience 81:9–16
Sellmayer A, Danesch U, Weber PC (1997) Modulation of the expression of early genes by polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 57:353–357
Bazan NG (2005) Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol 32:89–103
Ikemoto A, Kobayashi T, Emoto K, Umeda M, Watanabe S, Okuyama H (1999) Effects of docosahexaenoic and arachidonic acids on the synthesis and distribution of aminophospholipids during neuronal differentiation of PC12 cells. Arch Biochem Biophys 364:67–74
Stefanovic B, Bosetti F, Silva AC (2006) Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage 32:23–32
Adibhatla RM, Hatcher JF (2008) Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep 41:560–567
Acknowledgments
This research was supported entirely by the Intramural Program of the National Institute on Aging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HJ., Bazinet, R.P., Rapoport, S.I. et al. Brain Arachidonic Acid Cascade Enzymes are Upregulated in a Rat Model of Unilateral Parkinson Disease. Neurochem Res 35, 613–619 (2010). https://doi.org/10.1007/s11064-009-0106-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0106-6