Abstract
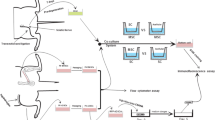
To investigate the morphological differences among acellular rat nerve scaffolds processed by different chemical methods and compare the biocompatibility between rat nerve grafts processed by different chemical methods and rat adipose-derived stem cells in vitro. Acellular rat sciatic nerve scaffolds processed by two different chemical methods (the Sondell method and the optimized method) and normal rat sciatic nerves were used as control. The structure and components of nerve scaffold were observed under microscopy, the degrees of decellularization and demyelination of nerve scaffold and integrity of nerve fiber tubes were assessed. The rat adipose-derived stem cells growth and adherence on scaffold were studied by scanning electron microscopy, the activity and adhesive ratio of rat adipose-derived stem cells in the nerve scaffold were compared. The basal lamina tubes and the extracellular matrix in the epineurium and perineurium in the nerve graft of optimized method were better preserved than the nerve graft of the Sondell method. After co-cultured with scaffolds, the difference of cell activity between three groups (two cell–scaffold combinations and control group) at the same observation time were not statistically significant (P > 0.05),the adhesive ratio of rat adipose-derived stem cells in the scaffold of the optimized method was better than that of the Sondell method. The scaffold of the optimized method is more effective than the scaffold of the Sondell method for peripheral nerve tissue engineering.


Similar content being viewed by others
References
Neubauer D, Graham JB, Muir D. Nerve grafts with various sensory and motor fiber compositions are equally effective for the repair of a mixed nerve defect. Exp Neurol. 2010;223:203–6.
Inada Y, Hosoi H, Yamashita A, et al. Regeneration of peripheral motor nerve gaps with a polyglycolic acid-collagen tube: technical case report. Neurosurgery. 2007;61:E1105–7.
Zhao Z, Wang Y, Peng J, et al. Repair of nerve defect with acellular nervegraft supplemented by bone marrow stromal cells in mice. Microsurgery. 2011;31:388–94.
Jeon WJ, Kang JW, Park JH, et al. Clinical application of inside-out vein grafts for the treatment of sensory nerve segmental defect. Microsurgery. 2011;31:268–73.
Nishimura T, Ueno T, Nakatsu H, Oga A, Kobayashi S, Oka M. In vivo motility evaluation of the grafted gastric wall with small intestinal submucosa. Tissue Eng Part A. 2010;16:1761–8.
Nishimura T, Ueno T, Nakatsu H, Oga A, Kobayashi S, Oka M. Chemically extracted acellular muscle: a new potential scaffold for spinal cord injury repair. J Biomed Mater Res A. 2012;100:578–87.
Itoh S, Yamaguchi I, Suzuki M, et al. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003;993:111–23.
Kang KN, Lee JY, da Kim Y, et al. Regeneration of completely transected spinal cord using scaffold of poly (d,l-lactide-co-glycolide)/small intestinal submucosa seeded with rat bone marrow stem cells. Tissue Eng Part A. 2011;17:2143–52.
Campbell GR, Campbell JH. Development of tissue engineered vascular grafts. Curr Pharm Biotechnol. 2007;8:43–50.
Tamerler C, Sarikaya M. Molecular biomimetics: utilizing nature’s molecular ways in practical engineering. Acta Biomater. 2007;3:289–99.
Nagao RJ, Lundy S, Khaing ZZ, Schmidt CE. Functional characterization of optimized acellular peripheral nerve graft in a rat sciatic nerve injury model. Neurol Res. 2011;33:600–8.
Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207:163–70.
Pfister LA, Papaloïzos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65–82.
Karlsson M, Johansson F, Kanje M. Polystyrene replicas of neuronal basal lamina act as excellent guides for regenerating neurites. Acta Biomater. 2011;7:2910–8.
Sun M, Wang X, Zhao B. Quality estimation and influence factors of the larger chemically acellular nerve allografts in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:779–82.
Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54.
Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–51.
Planat-Bénard V, Menard C, André M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–9.
Krajewska M, Krajewski S, Epstein JI, et al. Immunohistochemical analysis of bcl 2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–76.
Lynam D, Bednark B, Peterson C, Welker D, Gao M, Sakamoto JS. Precision microchannel scaffolds for central and peripheral nervous system repair. J Mater Sci Mater Med. 2011;22:2119–30.
Steinhaus S, Stark Y, Bruns S, et al. Polysialic acid immobilized on silanized glass surfaces: a test case for its use as a biomaterial for nerve regeneration. J Mater Sci Mater Med. 2010;21:1371–8.
Crapo PM, Medberry CJ, Reing JE, et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33:3539–47.
Freytes DO, Rundell AE, Vande Geest J, Vorp DA, Webster TJ, Badylak SF. Analytically derived material properties of multilaminated extracellular matrix devices using the ball-burst test. Biomaterials. 2005;26:5518–31.
Donzelli R, Maiuri F, Piscopo GA, de Notaris M, Colella A, Divitiis E. Role of extracellular matrix components in facial nerve regeneration: an experimental study. Neurol Res. 2006;28:794–801.
Dodla MC, Bellamkonda RV. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46.
Frerichs O, Fansa H, Schicht C, Wolf G, Schneider W, Keilhoff G. Reconstruction of peripheral nerves using acellular nerve grafts with implanted cultured Schwann cells. Microsurgery. 2002;22:311–5.
Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346–58.
Flynn L, Prestwich GD, le Semp JL, Woodhouse KA. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834–42.
Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cell. Exp Neurol. 2004;187:319–28.
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183:355–66.
Kang SK, Shin MJ, Jung JS, Kim YG, Kim KH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15:583–94.
Steed MB, Mukhatyar V, Valmikinathan C, Bellamkonda RV. Advances in bioengineered conduits for peripheralnerve regeneration. Atlas Oral Maxillofacc Surg Clin N Am. 2011;19:119–30.
Chalfoun CT, Wirth GA, Evans GR. Tissue engineered nerve constructs: where do we stand? J Cell Mol Med. 2006;10:309–17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, S., Zheng, Y., Cai, Q. et al. Comparison of morphology and biocompatibility of acellular nerve scaffolds processed by different chemical methods. J Mater Sci: Mater Med 25, 1283–1291 (2014). https://doi.org/10.1007/s10856-014-5150-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5150-3