Abstract
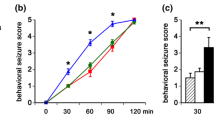
Temporal lobe epilepsy is characterized by spontaneous recurrent seizures (SRS) and associated with behavioral problems. However, the molecular mechanisms underlying these problems are not yet clear. In this study, kainic acid (KA) was systemically administered to immature male Wistar rats to induce SRS. The behavior of the immature rats was evaluated with a water maze, elevated-plus mazes, and open field tests. The expression patterns of synaptophysin, SNAP-25, and synaptotagmin 1 (Syt 1) were examined by reverse-transcriptase polymerase chain reaction (RT-PCR) and Western blot analysis. KA-treated rats with SRS demonstrated learning and memory deficits, reduced anxiety, and increased locomotor activity, compared with placebo-treated rats and KA-treated rats without SRS. No neuronal cell loss was observed in the hippocampus 6 weeks after exposure to KA. However, RT-PCR and Western blot analyses revealed decreased synaptophysin, SNAP-25, and Syt 1 expression in KA-treated rats with SRS. Synaptophysin, SNAP-25, and Syt1 expression levels were found to be positively correlated with learning and memory but negatively correlated with anxiety and locomotor activity. These data suggested that SRS may induce changes in synaptophysin, SNAP-25, and Syt1 expression and may be functionally related to SRS-induced behavioral deficits.





Similar content being viewed by others
Abbreviations
- KA:
-
Kainic acid
- SRS:
-
Spontaneous recurrent seizures
- Syt 1:
-
Synaptotagmin 1
- TLE:
-
Temporal lobe epilepsy
- SE:
-
Status epilepticus
- AD:
-
Alzheimer’s disease
References
Austin JK, Dunn DW (2002) Progressive behavioral changes in children with epilepsy. Prog Brain Res 135:419–427
Bennett JC, McRae PA, Levy LJ, Frick KM (2006) Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem 85:139–152
Bohbot VD, Corkin S (2006) Posterior parahippocampal place learning in H.M. Hippocampus 17:863–872
Bolanos AR, Sarkisian M, Yang Y, Hori A, Helmers SL, Mikati M, Tandon P, Stafstrom CE, Holmes GL (1998) Comparison of valproate and phenobarbital treatment after status epilepticus in rats. Neurology 51:41–48
Braun JE, Madison DV (2000) A novel SNAP25–caveolin complex correlates with the onset of persistent synaptic potentiation. J Neurosci 20:5997–6006
Chang YC, Huang AM, Kuo YM, Wang ST, Chang YY, Huang CC (2003) Febrile seizures impair memory and cAMP response-element binding protein activation. Ann Neurol 54:706–718
Chen GH, Wang YJ, Qin S, Yang QG, Zhou JN, Liu RY (2007) Age-related spatial cognitive impairment is correlated with increase of synaptotagmin 1 in dorsal hippocampus in SAMP8 mice. Neurobiol Aging 28:611–618
Costantin L, Bozzi Y, Richichi C, Viegi A, Antonucci F, Funicello M, Gobbi M, Mennini T, Rossetto O, Montecucco C, Maffei L, Vezzani A, Caleo M (2005) Antiepileptic effects of botulinum neurotoxin E. J Neurosci 25:1943–1951
Daly C, Ziff EB (1997) Post-transcriptional regulation of synaptic vesicle protein expression and the developmental control of synaptic vesicle formation. J Neurosci 17:2365–2375
Dickson JM, Wilkinson ID, Howell SJ, Griffiths PD, Grunewald RA (2006) Idiopathic generalised epilepsy: a pilot study of memory and neuronal dysfunction in the temporal lobes, assessed by magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 77:834–840
Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature 410:41–49
Frankland PW, Bontempi B (2005) The organization of recent and remote memories. Nat Rev Neurosci 6:119–130
Fuentes-Santamaria V, Alvarado JC, Henkel CK, Brunso-Bechtold JK (2007) Cochlear ablation in adult ferrets results in changes in insulin-like growth factor-1 and synaptophysin immunostaining in the cochlear nucleus. Neuroscience 148:1033–1047
Gosso MF, de Geus EJ, van Belzen MJ, Polderman TJ, Heutink P, Boomsma DI, Posthuma D (2006) The SNAP-25 gene is associated with cognitive ability: evidence from a family-based study in two independent Dutch cohorts. Mol Psychiatry 11:878–886
Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL (2001) Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus 11:615–625
Hanaya R, Boehm N, Nehlig A (2007) Dissociation of the immunoreactivity of synaptophysin and GAP-43 during the acute and latent phases of the lithium–pilocarpine model in the immature and adult rat. Exp Neurol 204:720–732
Haut SR, Veliskova J, Moshe SL (2004) Susceptibility of immature and adult brains to seizure effects. Lancet Neurol 3:608–617
Helmstaedter C, Elger CE (2009) Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain 132:2822–2830
Helmstaedter C, Kockelmann E (2006) Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia 47(Suppl. 2):96–98
Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B (2006) Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 60:80–87
Hinz B, Becher A, Mitter D, Schulze K, Heinemann U, Draguhn A, Ahnert-Hilger G (2001) Activity-dependent changes of the presynaptic synaptophysin–synaptobrevin complex in adult rat brain. Eur J Cell Biol 80:615–619
Loscher W (2002) Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 50:105–123
Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T (2000) Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci 12:2252–2264
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
McHugh SB, Deacon RM, Rawlins JN, Bannerman DM (2004) Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci 118:63–78
Pauli E, Hildebrandt M, Romstock J, Stefan H, Blumcke I (2006) Deficient memory acquisition in temporal lobe epilepsy is predicted by hippocampal granule cell loss. Neurology 67:1383–1389
Racine R, Okujava V, Chipashvili S (1972) Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol 32:295–299
Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 7:103–117 (discussion 173–180)
Sayin U, Sutula TP, Stafstrom CE (2004) Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia 45:1539–1548
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20:6587–6593
Spellmann I, Müller N, Musil R, Zill P, Douhet A, Dehning S, Cerovecki A, Bondy B, Möller HJ, Riedel M (2008) Associations of SNAP-25 polymorphisms with cognitive dysfunctions in Caucasian patients with schizophrenia during a brief trail of treatment with atypical antipsychotics. Eur Arch Psychiatry Clin Neurosci 258:335–344
Südhof TC (2002) Synaptotagmins: why so many? J Biol Chem 277:7629–7632
Suh EC, Jung YJ, Kim YA, Park EM, Lee KE (2008) A beta 25–35 induces presynaptic changes in organotypic hippocampal slice cultures. Neurotoxicology 29:691–699
Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ (1997) Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol 56:933–944
Szyndler J, Piechal A, Blecharz-Klin K, Skórzewska A, Maciejak P, Walkowiak J (2006) Effect of kindled seizures on rat behavior in water Morris maze test and amino acid concentrations in brain structures. Pharmacol Rep 58:75–82
Tocco G, Bi X, Vician L, Lim IK, Herschman H, Baudry M (1996) Two synaptotagmin genes, Syt1 and Syt4, are differentially regulated in adult brain and during postnatal development following kainic acid-induced seizures. Brain Res Mol Brain Res 40:229–239
Xiao Z, Gong Y, Wang XF, Xiao F, Xi ZQ, Lu Y, Sun HB (2009) Altered expression of synaptotagmin I in temporal lobe tissue of patients with refractory epilepsy. J Mol Neurosci 38:193–200
Yang Y, Tandon P, Liu Z, Sarkisian MR, Stafstrom CE, Holmes GL (1998) Synaptic reorganization following kainic acid-induced seizures during development. Brain Res Dev Brain Res 107:169–177
Yang JW, Czech T, Felizardo M, Baumgartner C, Lubec G (2006) Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe epilepsy. Amino Acids 30:477–493
Yin S, Guan Z, Tang Y, Zhao J, Hong J, Zhang W (2005) Abnormal expression of epilepsy-related gene ERG1/NSF in the spontaneous recurrent seizure rats with spatial learning memory deficits induced by kainic acid. Brain Res 1053:195–202
Zeng K, Wang X, Wang Y, Yan Y (2009) Enhanced synaptic vesicle traffic in hippocampus of phenytoin-resistant kindled rats. Neurochem Res 34:899–904
Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA (2001) Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiat 58:655–662
Zhou JL, Shatskikh TN, Liu X, Holmes GL (2007) Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur J Neurosci 25:3667–3677
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Shandong, China (Y2007C170).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, FX., Sun, QJ., Zheng, XY. et al. Abnormal Expression of Synaptophysin, SNAP-25, and Synaptotagmin 1 in the Hippocampus of Kainic Acid-Exposed Rats with Behavioral Deficits. Cell Mol Neurobiol 34, 813–824 (2014). https://doi.org/10.1007/s10571-014-0068-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-014-0068-3