Abstract
Endogenous brain activity supports spontaneous human thought and shapes perception and behavior. Connectivity-based analyses of endogenous, or resting-state, functional magnetic resonance imaging (fMRI) data have revealed the existence of a small number of robust networks which have a rich spatial structure. Yet the temporal information within fMRI data is limited, motivating the complementary analysis of electrophysiological recordings such as electroencephalography (EEG). Here we provide a novel method based on multivariate time–frequency interdependence to reconstruct the principal resting-state network dynamics in human EEG data. The stability of network expression across subjects is assessed using resampling techniques. We report the presence of seven robust networks, with distinct topographic organizations and high frequency (∼5–45 Hz) fingerprints, nested within slow temporal sequences that build up and decay over several orders of magnitude. Interestingly, all seven networks are expressed concurrently during these slow dynamics, although there is a temporal asymmetry in the pattern of their formation and dissolution. These analyses uncover the complex temporal character of endogenous cortical fluctuations and, in particular, offer an opportunity to reconstruct the low dimensional linear subspace in which they unfold.





Similar content being viewed by others
References
Akalin Acar Z, Makeig S (2013) Effects of forward model errors on EEG source localization. Brain Topogr 26(3):378–396
Aquino KM, Schira MM, Robinson PA, Drysdale PM, Breakspear M (2012) Hemodynamic traveling waves in human visual cortex. PLoS Comput Biol 8(3):e1002435
Ashwin P, Chossat P (1998) Attractors for robust heteroclinic cycles with continua of connections. J Nonlinear Sci 8(2):103–129
Banerjee A, Tognoli E, Assisi C, Kelso J, Jirsa V (2008) Mode level cognitive subtraction (MLCS) quantifies spatiotemporal reorganization in large-scale brain topographies. NeuroImage 42(2):663–674
Beckmann C, DeLuca M, Devlin J, Smith S (2005) Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B 360(1457):1001–1013
Birn R, Diamond J, Smith MA, Bandettini P (2006) Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31(4):1536–1548
Biswal B, Yetkin F, Haughton V, Hyde J (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Boonstra TW, Daffertshofer A, Breakspear M, Beek JP (2007) Multivariate time–frequency analysis of electromagnetic brain activity during bimanual motor learning. NeuroImage 36(2):370–377
Breakspear M (2002) Nonlinear phase desynchronization in human electroencephalographic data. Human Brain Mapp 15(3):175–198
Britz J, Van De Ville D, Michel C (2010) Bold correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage 52(4):1162–1170
Brookes M, Woolrich M, Luckhoo H, Price D, Hale J, Stephenson M, Barnes G, Smith S, Morris P (2011) Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci USA 108(40):16783–16788
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Buzsaki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929
Cardoso JF (1997) Infomax and maximum likelihood for blind source separation. IEEE Signal Process Lett 4(4):112–114
Carter G (1987) Coherence and time delay estimation. Proc IEEE 75(2):236–255
Cole D, Smith S, Beckmann C (2010) Advances and pitfalls in the analysis and interpretation of resting-state fMRI data. Front Syst Neurosci 4:1–15
Daffertshofer A, Lamoth C, Meijer O, Beek P (2004) PCA in studying coordination and variability: a tutorial. Clin Biomech 19(4):415–428
Damoiseaux J, Rombouts S, Barkhof F, Scheltens P, Stam C, Smith S, Beckmann C (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103(37):13848–13853
Daunizeau J, Friston K, Kiebel S (2009) Variational bayesian identification and prediction of stochastic nonlinear dynamic causal models. Physica D 238(21):2089–2118
De Pasquale F, Della Penna S, Snyder A, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani G, Corbetta M (2010) Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci USA 107(13):6040–6045
Deco G, Jirsa V (2012) Ongoing cortical activity at rest: criticality, multistability, and ghost attractors. J Neurosci 32(10):3366–3375
Deco G, Jirsa V, McIntosh A, Sporns O, Kötter R (2009) Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci USA 106(29):12207–12212
Dehghani N, Bédard C, Cash S, Halgren E, Destexhe A (2010) Comparative power spectral analysis of simultaneous elecroencephalographic and magnetoencephalographic recordings in humans suggests non-resistive extracellular media. J Comput Neurosci 29(3):405–421
Efron B, Petrosian V (1999) Nonparametric methods for doubly truncated data. J Am Stat Assoc 94(447):824–834
Engel A, König P, Kreiter A, Singer W (1991) Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science 252(5009):1177–1179
Fein G, Raz J, Brown F, Merrin E (1988) Common reference coherence data are confounded by power phase effects. Electroencephalogr Clin Neurophysiol 69(6):581–584
Fleuriet J, Goffart L (2012) Saccadic interception of a moving visual target after a spatiotemporal perturbation. J Neurosci 32(2):452–461
Fox M, Raichle M (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711
Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102(27):9673–9678
Fox M, Snyder A, Vincent J, Raichle M (2007) Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56(1):171–184
Freyer F, Aquino K, Robinson PA, Ritter P, Breakspear M (2009) Bistability and non-gaussian fluctuations in spontaneous cortical activity. J Neurosci 29(26):8512–8524
Freyer F, Roberts J, Becker R, Robinson P, Ritter P, Breakspear M (2011) Biophysical mechanisms of multistability in resting-state cortical rhythms. J Neurosci 31(17):6353–6361
Freyer F, Roberts J, Ritter P, Breakspear M (2012) A canonical model of multistability and scale-invariance in biological systems. PLoS Comput Biol 8(8):e1002634
Fries P (2005) A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9(10):474–480
Friston K (2002) Functional integration and inference in the brain. Prog Neurobiol 68(2):113–143
Friston K, Li B, Daunizeau J, Stephan K (2011) Network discovery with dcm. NeuroImage 56(3):1202–1221
Ganzetti M, Mantini D (2013) Functional connectivity and oscillatory neuronal activity in the resting human brain. Neuroscience 240:297–309
Ghosh A, Rho Y, McIntosh A, Kötter R, Jirsa V (2008) Noise during rest enables the exploration of the brain’s dynamic repertoire. PLoS Comput Biol 4(10):e1000196
Greicius M, Krasnow B, Reiss A, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100(1):253–258
Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R (2001) Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 98(2):694–699
He B, Raichle M (2009) The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci 13(7):302–309
Heitmann S, Gong P, Breakspear M (2012) A computational role for bistability and traveling waves in motor cortex. Front Comput Neurosci 6:1–15
Hillebrand A, Barnes G, Bosboom J, Berendse H, Stam C (2012) Frequency-dependent functional connectivity within resting-state networks: an atlas-based MEG beamformer solution. NeuroImage 59(4):3909–3921
Hipp J, Hawellek D, Corbetta M, Siegel M, Engel A (2012) Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci 15(6):884–890
Honey CJ, Kötter R, Breakspear M, Sporns O (2007) Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA 104(24):10240–10245
Jin SH, Jeong W, Seol J, Kwon J, Chung C (2013) Functional cortical hubs in the eyes-closed resting human brain from an electrophysiological perspective using magnetoencephalography. PLoS ONE 8(7):e68192
Khader P, Schicke T, Röder B, Rösler F (2008) On the relationship between slow cortical potentials and bold signal changes in humans. Int J Psychophysiol 67(3):252–261
Kobayashi T, Misaki K, Nakagawa H, Madokoro S, Ihara H, Tsuda K, Umezawa Y, Murayama J, Isaki K (1999) Non-linear analysis of the sleep EEG. Psychiatry Clin Neurosci 53(2):159–161
Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K (2003) EEG-correlated fMRI of human alpha activity. NeuroImage 19(4):1463–1476
Lehmann D, Michel C (1989) Intracerebral dipole sources of EEG FFT power maps. Brain Topography 2(1–2):155–164
Lewis C, Baldassarre A, Committeri G, Romani G, Corbetta M (2009) Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA 106(41):17558–17563
Linkenkaer-Hansen K, Nikouline V, Palva J, Ilmoniemi R (2001) Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci 21(4):1370–1377
Mantini D, Perrucci M, Del Gratta C, Romani G, Corbetta M (2007) Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104(32):13170–13175
Mason M, Norton M, Van Horn J, Wegner D, Grafton S, Macrae C (2007) Wandering minds: the default network and stimulus-independent thought. Science 315(5810):393–395
McIntosh A, Lobaugh N (2004) Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage 23(suppl. 1):S250–S263
Mehrkanoon S, Breakspear M, Daffertshofer A, Boonstra TW (2013) Non-identical smoothing operators for estimating time–frequency interdependence in electrophysiological recordings. EURASIP J Adv Signal Process 2013(73):1–16
Miller K, Weaver K, Ojemann J (2009) Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci USA 106(29):12174–12177
Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A (2003) Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage 20(1):145–158
Musso F, Brinkmeyer J, Mobascher A, Warbrick T, Winterer G (2010) Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. NeuroImage 52(4):1149–1161
Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld M, Kramer U, Arieli A, Fried I, Malach R (2008) Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci 11(9):1100–1108
Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M (2004) Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol 115(10):2292–2307
Nunez P, Srinivasan R (2006) A theoretical basis for standing and traveling brain waves measured with human eeg with implications for an integrated consciousness. Clin Neurophysiol 117(11):2424–2435
Perdikis D, Huys R, Jirsa V (2011) Complex processes from dynamical architectures with time-scale hierarchy. PLoS ONE 6(2):e16589
Rabinovich M, Huerta R, Varona P, Afraimovich V (2008) Transient cognitive dynamics, metastability, and decision making. PLoS Comput Biol 4(5):e1000072
Raichle M (2010) Two views of brain function. Trends Cogn Sci 14(4):180–190
Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G (2001) A default mode of brain function. Proc Natl Acad Sci USA 98(2):676–682
Ritter P, Moosmann M, Villringer A (2009) Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI−BOLD signal in primary somatosensory and motor cortex. Human Brain Mapp 30(4):1168–1187
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52(3):1059–1069
Schultze-Kraft M, Becker R, Breakspear M, Ritter P (2011) Exploiting the potential of three dimensional spatial wavelet analysis to explore nesting of temporal oscillations and spatial variance in simultaneous EEG-fMRI data. Prog Biophys Mol Biol 105(1-2):67–79
Smith S, Fox P, Miller K, Glahn D, Fox P, Mackay C, Filippini N, Watkins K, Toro R, Laird A, Beckmann C (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA 106(31):13040–13045
Sporns O (2010) Networks of the brain. The MIT Press, Massachusetts Institute of Technology, Cambridge
Stam C, Nolte G, Daffertshofer A (2007) Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Human Brain Mapp 28(11):1178–1193
Tass P, Rosenblum M, Weule J, Kurths J, Pikovsky VJ A and, Schnitzler A, Freund HJ (1998) Detection of n:m phase locking from noisy data: application to magnetoencephalography. Phys Rev Lett 81(15):3291–3294
Van De Ville D, Britz J, Michel C (2010) EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proc Natl Acad Sci USA 107(42):18179–18184
Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001) The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2(4):229–239
Womelsdorf T, Johnston K, Vinck M, Everling S (2010) Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci USA 107(11):5248–5253
Acknowledgments
This research was supported by the ARC Thinking Systems grant TS0669860; the National Health and Medical Research Council; BrainNRG collaborative award JSMF22002082, and the Netherlands Organization for Scientific Research (NWO #45110-030). The authors wish to thank Angela Langdon and James Roberts for their comments on a draft manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Multivariate Decomposition
Interdependences between channel pairs were assessed in the frequency domain. Spectral decomposition of EEG signals were obtained using the Fourier transform of sliding time windows as
where \({\bf j}=\sqrt{-1}, \phi_x[n,k]\) and ϕ y [n, k] denote the phase-spectra, S x [n, k] denotes the short-time Fourier transform of a discrete-time signal x[p] in the nth time-window, w n [l]. The w[l] is the unit energy window function and L is the total number of data samples in each time-window. The asterisk (*) denotes the complex conjugate. Time–frequency interdependence was assessed between all channel pairs. The total number of channel pairs is given by
where c = 61 denotes the number of EEG channels.
A single multidimensional array for each subject \(j=1,2, \ldots ,7\) and epoch i = 1, 2, 3 was constructed as
where \(\hat{\theta}_{I_{(r,s)}}[n,k]\) denotes the imaginary part of the time–frequency interdependence expressed in Eq. (1) between channels r and s. The symbol \(\dagger\) denotes array elements that, due to the undirected nature of Eq. (1) are symmetric across the major diagonal and these are not considered further. Since Eq. (1) is a lossless decomposition of the EEG data, the elements in the array \(\hat{\Upxi}_{i,j_{(r,s)}}[n,k]\) will inevitably be mutually correlated and contain considerable redundancies.
PCA was implemented through an eigen-decomposition of the covariance matrix of the array \(\hat{\Upxi}_{i,j_{(r,s)}}[n,k]. \) Since \(\hat{\Upxi}_{i,j_{(r,s)}}[n,k]\) has three factors (or dimensions) of time (n), frequency (k), and channel-combinations (b), we reshaped \(\hat{\Upxi}_{i,j_{(r,s)}}[n,k]\) into a 2-D array \(\Uptheta_{i,j}[nk,b], \quad nk=1,2, \ldots ,N\times K; \quad b=1,2, \ldots ,B, \) where B denotes the maximum number of possible channel combinations as defined in Eq. (5). That is, we reorganized the dimensions of \(\hat{\Upxi}_{i,j_{(r,s)}}[n,k]\) as time–frequency (N × K)-by-channel-combinations (B), expressed in matrix format \(\Uptheta_{i,j_{(nk\times n)}}. \) We then obtained the resting-state modes from the eigen-decomposition of the covariance matrix of \(\Uptheta_{i,j}[nk,b]\) as
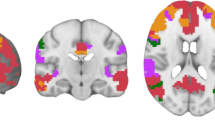
where \({\mathbb{E}}\) denotes the mathematical expectation, T the matrix transpose and, as above, i denotes each 4-min epoch within each subject j. E i,j is the B × B orthogonal matrix whose columns are the unit-norm eigenvectors of the covariance matrix \(\hat{R}_{\Uptheta\Uptheta_{i,j}}, \) and D i,j the diagonal matrix representing the eigenvalues of \(\hat{R}_{\Uptheta\Uptheta_{i,j}}, \) such that \(\hbox{diag}\Big(D_{i,j}\Big)=\{d_{i,j}^1>d_{i,j}^2> \ldots >d_{i,j}^B\}\) represents the variance of each putative resting-state mode. The eigenvectors of \(\hat{R}_{\Uptheta\Uptheta_{i,j}}\) correspond to the edges of the resting-state networks for the ith epoch in the jth subject. That is, each B × 1 eigenvector contains the contributions of every channel combination (i.e., edges) to that specific mode and hence reflects the spatial topology of each network. We considered the first 100 modes (columns of E i,j ) for further analysis. After realignment, this yields 100 modes for each epoch i and subject j as
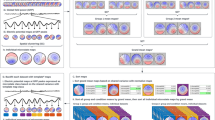
Jackknifing revealed that seven resting-state networks were robustly expressed across subjects and epochs. These networks were considered for further analysis. Although the variance between epochs can be used to assess the inter-subject (or test-retest) reliability, we focus on between-subject reliability and hence average across epochs, which yields
where, as before \(j=1,2, \ldots ,7\) runs across the seven subjects and \(m=1,2, \ldots ,7\) denotes the seven robust resting-state networks. Hence E[b, m](B × 7 × 7), D[m](7 × 7), and Z[nk,m](NK × 7 × 7) represent the 7 robust resting-state modes (eigenvectors), explained variances (eigenvalues), and projection time–frequency spectra for 7 subjects, respectively. For example, \(Z_{1}[nk,2]=\Uptheta_{1}[nk,b]E_{1}[b,2]\) is the projection time–frequency spectra for the second network in subject 1. The matrix E[b, m] contains the functional connectivity structure of each mode that can be visualized in terms of nodes (i.e., EEG electrode sites) or edges (i.e., the weighted links between nodes). After reshaping, the time–frequency spectra Z j,m [n, k] contain the spectral content and the temporal fluctuations of each robust networks.
Appendix 2: Temporal Expression of Robust Networks
We further assessed the networks’ temporal dynamics by estimating the Hurst exponent \(\hat{H}_{j,m}=\frac{\hbox{log}\it{(R_{j,m}/S_{j,m})}}{\hbox{log}(N)}, \) where R j,m /S j,m denotes the so-called range statistics: R j,m is the difference between the maximum and minimum deviation from the mean, and S j,m is the standard deviation over total number of N samples for the jth subject and the mth network. The Hurst exponent is a measure of the extent of long-range dependence, i.e., for \(\hat{H}_{j,m}=0.5\) increments that are uncorrelated, whereas \(0.5<\hat{H}_{j,m}<1\) indicates the presence of long-range dependencies or persistence (Kobayashi et al. 1999).
The Hurst exponent of the temporal fluctuations \(\acute{\sigma}^2_{j,m}[n]\) of each network expressed in Eq. (3), is given by
where
where ϑ j,m denotes the deviation of mth network from its mean value \(\bar{\acute{\sigma}}_{j,m}, \) and non-overlapped samples are chosen as M = N/4.
We finally study the relative temporal expression of resting-state networks by determining the cross-correlation between their time series \(\acute{\sigma}^{2}_{j,m}[n]. \) To ensure normality, the time-series \(\acute{\sigma}^{2}_{j,m}[n]\) were first log transformed and then low-pass filtered at frequency 0.1 Hz. The cross-correlation between the times series of two networks of u and v is given as a function of a variable time lag τ by
To extract the component of the cross-correlation functions that is not invariant to time reversal, we determine the asymmetric components of C j_(u,v)[τ] by reconstructing
where C j_(u,v)[τ] is the cross-correlation function of the time series \(\acute{\sigma}^{2}_{j,m}[n]\) and T the transpose. The signs of the slope of the asymmetric component at zero lag, \(\acute{C}_{j_{(u,v)}}[0], \) were used to represent the relative leading (positive sign) and lagging (negative sign) position of networks u and v. Examining all pairs of networks in each subject allows a temporal rank order (sequence) to be obtained.
Confidence intervals for the asymmetric component of the cross correlation were estimated using a non-parametric permutation approach. To this end, 1000 surrogate time series were constructed by random permutation of the log transformed low-pass filtered network time series. The asymmetric component of the cross-correlation functions Eq. (13) derived from these surrogate time series hence represent trivial non-zero fluctuations of the magnitude expected from data of that string length and amplitude distribution but with no further structure of interest. Two-sided 95 % confidence intervals were estimated by rank ordering this null distribution and choosing the 25th and 975th values. This process was repeated independently for each of the seven robust networks in all 3 epochs of all subjects.
Rights and permissions
About this article
Cite this article
Mehrkanoon, S., Breakspear, M. & Boonstra, T.W. Low-Dimensional Dynamics of Resting-State Cortical Activity. Brain Topogr 27, 338–352 (2014). https://doi.org/10.1007/s10548-013-0319-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-013-0319-5