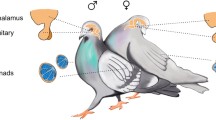
Abstract
Sex steroids influence a diversity of neural and behavioral endpoints in birds, including some that have little to do with reproduction per se. Recent advances in neurochemistry and molecular biology further indicate that the avian brain comprises a network of unique sex steroid microenvironments. Factors involved in steroid synthesis and metabolism are present in the avian brain with expression levels that vary from region to region and with activities that are, in some cases, subject to regulation over relatively slow or rapid time intervals. Advances in our ability to (1) isolate steroids from brain tissue and (2) precisely measure their concentrations reveal how steroid levels vary spatially and temporally. A full appreciation of sex steroid effects on the avian brain require not only measures of hormones in the blood but also an understanding of the numerous and varied mechanisms whereby the brain creates such a heterogeneous steroidal environment.
Similar content being viewed by others
References
Acharya KD, Veney SL (2012) Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol 72:1433–1446. doi:10.1002/dneu.22004
Bailey DJ, Ma C, Soma KK, Saldanha CJ (2013) Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology 154:4707–4714. doi:10.1210/en.2013-1684
Ball GF, Balthazart J (2009) Neuroendocrine regulation of reproductive behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (eds) Hormones, brain and behavior, vol 2. Academic Press, San Diego, pp 855–895
Balthazart J, Ball GF (2006) Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29:241–249
Balthazart J, Ball GF (eds) (2013) Brain aromatase, estrogens and behavior. Behavioral neuroendocrinology. Oxford University Press, Oxford
Baulieu EE (1998) Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 23:963–987
Carlisle HJ, Hales TG, Schlinger BA (1998) Characterization of neuronal zebra finch GABA(A) receptors: steroid effects. J Comp Physiol A 182:531–538
Chao A, Schlinger BA, Remage-Healey L (2011) Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat 5:57. doi:10.3389/fnana.2011.00057
Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, Soma KK (2011) Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol 23:742–753. doi:10.1111/j.1365-2826.2011.02170.x
Cornil CA, Charlier TD (2010) Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol 22:664–673. doi:10.1111/j.1365-2826.2010.02023.x
Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ (2012) Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology 153:2562–2567. doi:10.1210/en.2011-2159
Fusani L, Donaldson Z, London SE, Fuxjager MJ, Schlinger BA (2014) Expression of androgen receptor in the brain of a sub-oscine bird with an elaborate courtship display. Neurosci Lett 578:61–65. doi:10.1016/j.neulet.2014.06.028
Gahr M, Flugge G, Guttinger HR (1987) Immunocytochemical localization of estrogen-binding neurons in the songbird brain. Brain Res 402:173–177
Hutchison JB, Steimer T (1981) Brain 5beta-reductase: a correlate of behavioral sensitivity to androgen. Science 213:244–246
Hutchison JB, Steimer T (1984) Androgen metabolism in the brain: behavioural correlates. Prog Brain Res 61:23–51
Landys MM, Goymann W, Soma KK, Slagsvold T (2013) Year-round territorial aggression is independent of plasma DHEA in the European nuthatch sitta europaea. Horm Behav 63:166–172. doi:10.1016/j.yhbeh.2012.10.002
London SE, Monks DA, Wade J, Schlinger BA (2006) Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology 147:5975–5987. doi:10.1210/en.2006-0154
London SE, Remage-Healey L, Schlinger BA (2009) Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol 30:302–314. doi:10.1016/j.yfrne.2009.05.001
Metzdorf R, Gahr M, Fusani L (1999) Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol 407:115–129
Miller WL (1988) Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318
Newman AE, Soma KK (2009) Corticosterone and dehydroepiandrosterone in songbird plasma and brain: effects of season and acute stress. Eur J Neurosci 29:1905–1914. doi:10.1111/j.1460-9568.2009.06748.x
Newman AE, Pradhan DS, Soma KK (2008) Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology 149:2537–2545. doi:10.1210/en.2007-1363
Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ (2004) Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm Behav 45:250–258
Peterson RS, Yarram L, Schlinger BA, Saldanha CJ (2005) Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc R Soc B Biol Sci 272:2089–2096
Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK (2010) Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav 57:381–389. doi:10.1016/j.yhbeh.2010.01.008
Remage-Healey L, Joshi NR (2012) Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci 32:8231–8241. doi:10.1523/JNEUROSCI.1114-12.2012
Remage-Healey L, Oyama RK, Schlinger BA (2009) Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol 21:191–199. doi:10.1111/j.1365-2826.2009.01820.x
Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA (2010) Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Nat Acad Sci USA 107:3852–3857. doi:10.1073/pnas.0906572107
Remage-Healey L, Dong S, Maidment NT, Schlinger BA (2011) Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci 31:10034–10038. doi:10.1523/JNEUROSCI.0566-11.2011
Remage-Healey L, Dong SM, Chao A, Schlinger BA (2012) Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol 107:1621–1631. doi:10.1152/jn.00749.2011
Rensel MA, Salwiczek L, Roth J, Schlinger BA (2013) Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem 100:41–47. doi:10.1016/j.nlm.2012.12.005
Rensel MA, Comito D, Kosarussavadi S, Schlinger BA (2014) Region-specific neural corticosterone patterns differ from plasma in a male songbird. Endocrinology 155:3572–3581. doi:10.1210/en.2014-1231
Saldanha CJ, Popper P, Micevych PE, Schlinger BA (1998) The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav 34:85–97
Saldanha CJ, Clayton NS, Schlinger BA (1999) Androgen metabolism in the juvenile oscine forebrain: a cross-species analysis at neural sites implicated in memory function. J Neurobiol 40:397–406
Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA (2000) Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol 423:619–630
Saldanha CJ, Remage-Healey L, Schlinger BA (2011) Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32:532–549. doi:10.1210/er.2011-0004
Schlinger BA, Brenowitz EA (2009) Neural and hormonal control of birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (eds) Hormones, brain and behavior, vol 2. Academic Press, San Diego, pp 897–941
Schlinger BA, Callard GV (1987) A comparison of aromatase, 5 alpha-, and 5 beta- reductase activities in the brain and pituitary of male and female quail (C. c. japonica). J Exp Zool 242:171–180
Schlinger BA, Remage-Healey L (2012) Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol 24:16–21. doi:10.1111/j.1365-2826.2011.02150.x
Schlinger BA, Remage-Healey L, Rensel M (2014) Establishing regional specificity of neuroestrogen action. Gen Comp Endocrinol 205:235–241. doi:10.1016/j.ygcen.2014.03.043
Schumacher M, Balthazart J (1987) Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res 422:137–148
Shah AH, Chin EH, Schmidt KL, Soma KK (2011) DHEA and estradiol levels in brain, gonads, adrenal glands, and plasma of developing male and female European starlings. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197:949–958. doi:10.1007/s00359-011-0655-4
Soma KK, Wingfield JC (2001) Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol 123:144–155
Soma KK, Bindra RK, Gee J, Wingfield J, Schlinger BA (1999) Androgen metabolizing enzymes show region-specific changes across the breeding season in the brain of a wild breeding song bird. J Neurobiol 41:176–188
Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ (2003) Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol 56:209–221
Soma KK, Alday NA, Schlinger BA (2004) DHEA metabolism by 3 beta-HSD in adult Zebra finch brain: sex difference and rapid effect of stress. Endocrinology 145:1668–1677
Stocco DM, Clark BJ (1996) Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244
Tam H, Schlinger BA (2007) Activities of 3B-HSD and aromatase in slices of the adult and developing Zebra Finch brain. Gen Comp Endocrinol 150:26–33
Taves MD, Schmidt KL, Ruhr IM, Kapusta K, Prior NH, Soma KK (2010) Steroid concentrations in plasma, whole blood and brain: effects of saline perfusion to remove blood contamination from brain. PLoS One 5:e15727. doi:10.1371/journal.pone.0015727
Taves MD, Ma C, Heimovics SA, Saldanha CJ, Soma KK (2011) Measurement of steroid concentrations in brain tissue: methodological considerations. Front Endocrinol (Lausanne) 2:39. doi:10.3389/fendo.2011.00039
Tremere LA, Jeong JK, Pinaud R (2009) Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci 29:5949–5963. doi:10.1523/JNEUROSCI.0774-09.2009
Tsutsui K, Matsunaga M, Miyabara H, Ukena K (2006) Neurosteroid biosynthesis in the quail brain: a review. J Exp Zool Part A Comp Exp Biol 305A:733–742
Tsutsui K, Inoue K, Miyabara H, Suzuki S, Ogura Y, Tobari Y, Haraguchi S (2009) Discovery of a novel avian neurosteroid, 7alpha-hydroxypregnenolone, and its role in the regulation of the diurnal rhythm of locomotor activity in Japanese quail. Gen Comp Endocrinol 163:117–122. doi:10.1016/j.ygcen.2009.04.005
Ukena K, Honda Y, Inai Y, Kohchi C, Lea RW, Tsutsui K (1999) Expression and activity of 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4-isomerase in different regions of the avian brain. Brain Res 818:536–542
Vanson A, Arnold AP, Schlinger BA (1996) 3B-Hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol 102:342–350
Wingfield JC (2005) Historical contributions of research on birds to behavioral neuroendocrinology. Horm Behav 48:395–402
Wingfield JC, Farner DS (1993) Endocrinology of reproduction in wild species. In: Farner DS, King JR, Parkes KC (eds) Avian biology, vol 9. Academic Press, San Diego, pp 163–327
Wingfield J, Hegner R, Dufty A, Ball G (1990) The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Acknowledgment
Supported by NIH MH061994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Matthysen.
Rights and permissions
About this article
Cite this article
Schlinger, B.A. Steroids in the avian brain: heterogeneity across space and time. J Ornithol 156 (Suppl 1), 419–424 (2015). https://doi.org/10.1007/s10336-015-1184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1184-7