Abstract
Reduced hippocampal GABAergic inhibition is acknowledged to be associated with epilepsy. However, there are no studies that had quantitatively compared the loss of various interneuron populations in different models of epilepsy. We tested a hypothesis that the more severe the loss of hippocampal interneurons, the more severe was the epilepsy. Epileptogenesis was triggered in adult rats by status epilepticus (SE) (56 SE, 24 controls) or by traumatic brain injury (TBI) (45 TBI, 23 controls). The total number of hippocampal parvalbumin (PARV), cholecystokinin (CCK), calretinin (CR), somatostatin (SOM), or neuropeptide Y (NPY) positive neurons was estimated using unbiased stereology at 1 or 6 months post-insult. The rats with TBI had no spontaneous seizures but showed increased seizure susceptibility. Eleven of the 28 rats (39 %) in the SE group had spontaneous seizures. The most affected hippocampal area after TBI was the ipsilateral dentate gyrus, where 62 % of PARV-immunoreactive (ir) (p < 0.001 compared to controls), 77 % of CR-ir (p < 0.05), 46 % of SOM-ir (p < 0.001), and 59 % of NPY-ir (p < 0.001) cells remained at 1 month after TBI. At 6 months post-TBI, only 35 % of PARV-ir (p < 0.001 compared to controls), 63 % of CCK-ir (p < 0.01), 74 % of CR-ir (p < 0.001), 55 % of SOM-ir (p < 0.001), and 51 % of NPY-ir (p < 0.001) cells were remaining. Moreover, the reduction in PARV-ir, CCK-ir, and CR-ir neurons was bilateral (all p < 0.05). Substantial reductions in different neuronal populations were also found in subfields of the CA3 and CA1. In rats with epilepsy after SE, the number of PARV-ir neurons was reduced in the ipsilateral CA1 (80 % remaining, p < 0.05) and the number of NPY-ir neurons bilaterally in the dentate gyrus (33–37 %, p < 0.01) and the CA3 (54–57 %, p < 0.05). Taken together, interneuron loss was substantially more severe, widespread, progressive, and included more interneuron subclasses after TBI than after SE. Interneurons responsible for perisomatic inhibition were more vulnerable to TBI than those providing dendritic inhibition. Unlike expected, we could not demonstrate any etiology-independent link between the severity of hippocampal interneuron loss and the overall risk of spontaneous seizures.
Similar content being viewed by others
Introduction
Reduction in the number of hippocampal inhibitory interneurons has been considered as a major pathology resulting in the development of unprovoked seizures both in experimental models and in human temporal lobe epilepsy (TLE) (Mathern et al. 1995; Wasterlain et al. 1996; Maglóczky and Freund 2005; Tóth et al. 2010). These data are largely based on findings from rodent models of epilepsy induced by status epilepticus (SE), which results in epilepsy in a large majority of animals, and on the analysis of tissue available from patients with different epileptogenic etiologies who underwent hippocampal resection due to drug-refractory epilepsy (Ojemann 1987; Mathern et al. 1995; Wasterlain et al. 1996; Maglóczky et al. 2000; Gorter et al. 2001; Andrioli et al. 2007; Tóth et al. 2010). The importance of hippocampal GABAergic interneurons in seizure control has been further emphasized by studies showing increased susceptibility to epileptic seizures in genetically modified mice with abnormal development of hippocampal GABAergic neurons (Vreugdenhil et al. 2003; Schwaller et al. 2004). Studies have also demonstrated that increasing hippocampal GABAergic function, for example, by neuropeptide Y (NPY) gene therapy (Noè et al. 2008), neurosteroids (Biagini et al. 2010), transplantation of GABAergic cells (Maisano et al. 2012), or by GABA-enhancing pharmacological treatments (Czuczwar and Patsalos 2001; Söderpalm 2002) results in the suppression of epileptiform activity and seizures.
There are several proposed mechanisms by which a reduction in GABAergic neurons can lead to epileptogenesis. Degeneration of cells immunoreactive for parvalbumin (PARV-ir) or cholecystokinin (CCK-ir) impairs inhibition in the perisomatic region or axon initial segment of the principal cells in the dentate gyrus and the hippocampus proper (DeFelipe 1999; Arellano et al. 2004). Reduction in interneurons immunoreactive for somatostatin (SOM-ir), neuropeptide Y (NPY-ir), or calretinin (CR-ir) can compromise the dendritic inhibition of principal cells or other inhibitory neurons (Mathern et al. 1995; Maglóczky and Freund 2005; Tóth et al. 2010). Moreover, loss of presynaptic GABAergic terminals has been shown to result in reduced transmitter release and abnormal expression patterns of GABAA-receptor subunits in post-synaptic neurons, which can also contribute to hyperexcitability (Zhang et al. 2007; Pavlov et al. 2011). Importantly, experimental and human epileptic hippocampi have shown that the axons of the remaining GABAergic neurons can also sprout, which can compensate the early post-injury impairment of inhibition (Mathern et al. 1997; Bausch 2005; Zhang et al. 2009; Maglóczky 2010). Moreover, abnormalities in GABAergic innervation can also result in abnormal tuning and synchronization of principal cells, which compromises cognitive function in epilepsy (Klausberger and Somogyi 2008).
Relatively few studies have, however, investigated whether the reduction in hippocampal GABAergic interneurons undisputedly leads to the occurrence of spontaneous seizures, or whether there is an association between the severity of interneuron loss and seizure susceptibility (Buckmaster and Dudek 1997; Arellano et al. 2004). Moreover, there is little information on the syndrome-specific vulnerability of various interneuron subclasses in the same hippocampus (Schwarzer et al. 1995; Sun et al. 2007; Long et al. 2011). As acquired epilepsy is apparently a progressive disease, a question arises whether the progressive degeneration of interneurons contributes to the progression of epilepsy (Mathern et al. 1996; Gorter et al. 2001; Pitkänen and Sutula 2002; Pitkänen and Lukasiuk 2011). Finally, it is currently unknown whether data on interneuron loss in one epileptogenic etiology can be extrapolated to another. Such information would be valuable when planning the development of novel treatments to combat epilepsies caused by different etiologies. To tackle these questions and to test a hypothesis that the occurrence as well as the severity of seizure susceptibility or epilepsy is associated with the magnitude of chronic hippocampal interneuron loss, we estimated the total number of neurons in five different subclasses of hippocampal GABAergic cells using unbiased stereology in two models of acquired focal epilepsy, status epilepticus (SE) and traumatic brain injury (TBI), in which the hippocampus is involved in seizure activity (Nissinen et al. 2000; Kharatishvili et al. 2006).
Materials and methods
The study design is summarized in Fig. 1. In both models, one group of animals was killed for histology at 1 month (Fig. 1b, d) and another at 6 months (Fig. 1a, c) post-insult.
Study design. The present study analyzed and compared two models of acquired focal epilepsy, in which epileptogenesis was triggered by traumatic brain injury (TBI) or status epilepticus (SE). a, b TBI was induced by lateral fluid-percussion injury (FPI) (Kharatishvili et al. 2006). a In the 6-month follow-up group, rats were implanted with cortical electrodes and exposed to a pentylenetetrazol (PTZ) seizure susceptibility test at 5.5 months post-TBI. Thereafter, they were continuously video-EEG monitored for 2 weeks to detect spontaneous seizures. At 6 months post-TBI they were perfused for histology. b One group of rats with TBI was perfused for histology at 1 month post-TBI. c, d Status epilepticus (SE) was induced by electrical stimulation of the amygdala (Nissinen et al. 2000). To obtain rats with or without epilepsy, animals received injections of diazepam at 2 and 6 h after the beginning of SE (Pitkänen et al. 2005). c The 6-month follow-up group underwent 2-week continuous video-EEG monitoring starting at 5.5 months post-SE to detect spontaneous seizures. At 6 months post-SE, they were perfused for histology. d One group of rats was perfused for histology at 1 month post-SE. Sham-operated controls were subjected to all procedures except for the induction of TBI or SE
Animals
A total of 163 adult male Sprague–Dawley rats (initial body weight 275 g; Harlan Netherlands B.V., Horst, the Netherlands) were used. All rats were individually housed in a controlled environment (temperature 22 ± 1 °C; humidity 50–60 %; lights on from 07:00 to 19:00 h). Pellet food and water were provided ad libitum. All animal procedures were conducted in accordance with the guidelines of the European Community Council Directives 86/609/EEC, and approved by the Committee for the Welfare of Laboratory Animals of the University of Kuopio and the Provincial Government of Kuopio, Finland.
Lateral fluid-percussion induced TBI
Induction of TBI
The procedure for induction of lateral fluid-percussion injury (FPI) has been described previously (McIntosh et al. 1989; Kharatishvili et al. 2006). In brief, animals (n = 45, from which 13 were in the 1-month group and 32 were in the 6-month group) were anesthesized with an intraperitoneal (i.p.) injection of a solution (6 ml/kg) containing sodium pentobarbital (58 mg/kg), chloral hydrate (60 mg/kg), magnesium sulfate (127.2 mg/kg), propylene glycol (42.8 %) and absolute ethanol (11.6 %), and placed in a Kopf stereotactic frame (David Kopf Instruments, Tujunga, CA, USA). The skull was exposed with a midline skin incision and extraction of the periosteum was made. The left temporal muscle was gently detached from the lateral ridge. A circular craniectomy (Ø 5 mm) was performed over the left parietal lobe midway between lambda and bregma in a way that dura mater remained intact. The edges of craniectomy were sealed with a modified Luer-Lock cap that was filled with saline while the calvaria were covered with dental acrylate (Selectaplus CN, Dentsply DeTrey GmbH, Dreieich, Germany). Lateral FPI was induced 90 min after the administration of anesthesia by connecting the rat to a fluid-percussion device (AmScien Instruments, Richmond, VA, USA) via a female Luer-Lock fitting. The mean severity of the impact was 3.25 ± 0.03 atm. Control animals (n = 23, from which 10 were in the 1-month group and 13 in the 6-month group) received anesthesia and all surgical procedures without lateral FPI.
Implantation of electrodes for video-EEG recording
In the 6-month TBI group, cortical electrodes were implanted around 5 months post-TBI (3 weeks before killing) as described by Kharatishvili et al. (2006). In brief, rats were anesthesized (see above) and placed to a stereotactic frame. Six cortical stainless steel screw electrodes (E363/20 Plastics One Inc., Roanoke, VA, USA) were implanted into the skull: one rostral to the craniectomy, one contralateral to the centre of the craniectomy, two bilaterally over the frontal cortex, and two bilaterally over the cerebellum (ground and reference electrodes). Electrodes were attached to a plastic pedestal and fixed on the skull with dental acrylate.
Pentylenetetrazol test
To assess post-TBI increase in seizure susceptibility, rats underwent a pentylenetetrazol (PTZ, 1,5-pentamethylenetetrazole) test under video-EEG recording at 1 week after electrode implantation. Animals were placed in transparent plexiglass cages (47 × 29 × 50 cm3) and connected to the video-EEG system via a 6-channel commutator (Plastics One, Inc., Roanoke, VA, USA) and shielded cables. Video-EEG recording was performed using a Nervus EEG recording system (sampling rate 200–256 Hz, high pass filter of 0.3 Hz and low pass filter of 100 Hz; Taugagreining, Iceland) that was connected to a Nervus magnus 32/8 amplifier (Taugagreining, Iceland), an SVT-S3000P Time Lapse 168 VCR (Hitachi, Japan), and a WV-CL350 Video Camera (Panasonic, Japan).
After a 3–4 h baseline video-EEG recording, animals were injected with PTZ (25 mg/kg, i.p., Sigma-Aldrich YA-Kemia Oy, Finland) dissolved in 0.9 % saline (final concentration 12.5 mg/ml) and continuously video-EEG monitored for 60 min. The EEG files were visually analyzed. The electrographic parameters assessed included (1) latency to the first spike (s), (2) latency to the first epileptiform discharge (ED) (s), (3) total number of spikes, (4) total number of EDs, (5) duration of EDs (s), (6) occurrence of electrographic seizures (yes/no), (7) latency to an electrographic seizure, and (8) duration of an electrographic seizure (s). An epileptiform spike was defined as a high-amplitude (twice baseline), short-lasting (20–70 ms), sharply contoured waveform followed by a low-voltage slow potential. ED was defined as a high-amplitude (at least twice the baseline) rhythmic discharge comprised a slow wave, spike-wave, and/or polyspike-wave components lasting <5 s. An electrographic seizure was defined as a high-amplitude (more than twice the baseline) rhythmic discharge with temporal evolution in wave morphology and amplitude, having a clear onset and offset, and lasting >5 s. Also, the occurrence of behavioral seizures during the first 60 min after PTZ administration was monitored.
Monitoring of spontaneous seizures
After the PTZ test, video-EEG recording was continued for 2 weeks (24/7) to detect spontaneous seizures (for methodology, see Nissinen et al. 2000). According to our previous data, all seizures that had hippocampal involvement were also detected with cortical electrodes (Nissinen et al. 2000). Each EEG file was analyzed manually by browsing through the EEG recording on the computer screen. If an electrographic seizure was observed, behavioral severity was also analyzed from the corresponding video-recording. A spontaneous electroencephalographic seizure was defined as high-amplitude rhythmic discharge that clearly represented a new pattern of tracing (repetitive spikes, spike-and-wave discharges, and slow waves) and lasted for at least 5 s. Epileptic events occurring with an interval less than 5 s without the EEG returning to baseline were defined as belonging to the same seizure.
Induction of status epilepticus
Implantation of electrodes
Methodology for induction of SE has been described in detail previously (Nissinen et al. 2000). In brief, anesthetized (see above) rats (n = 56, from which 14 were in the 1-month group and 42 in the 6-month group) were fixed to a stereotactic frame (lambda and bregma at the same horizontal level). To stimulate the lateral nucleus of left amygdala, a hole (Ø 3 mm) was drilled to the exposed skull at 3.6 mm posterior and 5.0 mm lateral to the bregma, according to the rat brain atlas of Paxinos and Watson (1986). A bipolar stimulation electrode (distance between the tips 0.5 mm) was lowered down to 6.5 mm ventral to the surface of the brain. Additionally, two unipolar screw electrodes were implanted bilaterally over the frontal cortex and two bilaterally over the cerebellum (ground and reference electrodes). Electrodes were connected to a plastic pedestal and fixed to the skull with dental acrylate. Animals were left to recover in their cages for 7–14 days before the induction of SE.
Induction of status epilepticus
Rats were placed into transparent plexiglass cages (one rat per cage) and video-EEG monitored via amygdaloidal and cortical electrodes for at least 30 min. Then, SE was induced by stimulating the left amygdala for 20–40 min with a 100 ms train of 1 ms biphasic square-wave pulses (400 μA from peak to peak) at 60 Hz with an interval of 0.5 s as described before (Nissinen et al. 2000). The progression of SE was followed with continuous video-EEG monitoring. To limit the later development of epilepsy to about 50 % of all animals, rats were treated with diazepam (i.p., Stesolid Novum, 5 mg/ml, Dumex-Alpharma, Denmark) at 2 h (20 mg/kg) and 6 h (10 mg/kg) after the onset of SE (Pitkänen et al. 2005). Control animals (n = 24, from which 10 were in the 1-month group and 14 in the 6-month group) underwent the same procedures without electrical stimulation.
Monitoring of spontaneous seizures
At the end of the 6-month post-SE survival period, animals underwent 24/7 video-EEG monitoring for 2 weeks to detect the occurrence of spontaneous seizures (see above).
Histology
Fixation
At 1 or 6 months post-TBI or post-SE, rats were deeply anesthetized and transcardially perfused according to the pH-shift protocol: 0.9 % sodium chloride solution (4 °C) for 2 min at the speed of 30–35 ml/min, followed by 4 % paraformaldehyde (PFA) in 0.1 M sodium acetate buffer, pH 6.5 (4 °C) for 10 min, and 4 % PFA in 0.1 M sodium borate, pH 9.5 (4 °C) for 15 min. Brains were immediately removed from the skull and postfixed in 4 % PFA in 0.1 M sodium borate, pH 9.5 (4 °C) for 6 h. Subsequently, brains were cryoprotected in 20 % glycerol in 0.02 M potassium phosphate buffered saline (KPBS, pH 7.4) for 36 h. Brains were blocked, frozen in dry ice for 15 min, and stored at −70 °C until further processed.
Processing of tissue
Brains were sectioned in coronal plane (1-in-8 series, 25 μm) with a sliding microtome (Leica SM 2000, Leica Microsystems Nussloch GmbH, Nussloch, Germany). The first series of sections was stored in 10 % formalin at room temperature and the rest in cryoprotectant tissue-collecting solution [TCS; 30 % ethylene glycol, 25 % glycerol in 0.05 M sodium phosphate buffer (PB), pH 7.4] at −20 °C until processed.
Thionin staining
The first series was stained with thionin to define the cytoarchitectonic borders of different brain areas. Sections were washed with 0.1 M PB, mounted on gelatin-coated slides and dried. Slides were then stained using the following protocol: 1 h in chloroform-absolute ethanol 1:1, 2 times 2 min in absolute ethanol, 2 min in 96 % ethanol, 2 min in 70 % ethanol, 2 min in 50 % ethanol, 2 min in dH2O, 10 s in 0.625 % thionin solution, 2 min in 50 % ethanol, 2 min in 70 % ethanol, 0.5–3 min in solution containing glacial acetic acid in 96 % ethanol, 2 min in 96 % ethanol, 2 min in absolute ethanol, 5 min in absolute ethanol, and 3 times 3 min in xylene. Finally, slides were coverslipped using DePeX® (BDH Chemical, Poole, United Kingdom) as a mounting medium.
Parvalbumin immunohistochemistry
For parvalbumin (PARV) immunohistochemistry, an adjacent series of free-floating sections was washed three times in 0.02 M KPBS, pH 7.4. To remove endogenous peroxidase activity, sections were incubated in 1 % H2O2 in 0.02 M KPBS at room temperature (RT) for 15 min. After washing six times in 0.02 M KPBS, non-specific binding was blocked by incubating the sections in 10 % normal horse serum (NHS) and 0.5 % Triton X-100 (TX-100) in 0.02 M KPBS at RT for 2 h. Sections were washed three times in 2 % NHS in 0.02 M KPBS and incubated for 3 days (4 °C) in a primary antibody solution containing mouse-monoclonal antibody raised against PARV (1:15,000, #235, Swant, Bellinzone, Switzerland), 1 % NHS, and 0.5 % Triton X-100 (TX-100) in 0.02 M KPBS. Sections were washed three times (2 % NHS in 0.02 M KPBS) and incubated in biotinylated horse anti-mouse IgG (1:200, Vector # BA-2000, Vector Laboratories, CA, USA), 1 % NHS and 0.3 % TX-100 in 0.02 M KPBS for 1 h at RT. Then, sections were washed three times (2 % NHS in 0.02 M KPBS) and incubated in avidin–biotin (1:100, PK-4000, Vector Laboratories) in 0.02 M KPBS for 45 min at RT. After three washes (2 % NHS in 0.02 M KPBS), sections were recycled back to the secondary antibody solution for 45 min at RT, washed, and incubated in avidin–biotin solution for 30 min at RT. After three washes (2 % NHS in 0.02 M KPBS), the secondary antibody was visualized with 0.1 % 3′,3′-diaminobenzidine (DAB, Pierce Chemicals, Rockford, IL, USA) and 0.08 % H2O2 in 0.02 M KPBS. Sections were washed three times in KPBS, once in 0.1 M PB, and mounted on gelatin-coated microscope slides. Slides were dried overnight at 37 °C. Subsequently, the reaction product was intensified with osmium (OsO4) thiocarbohydrate according to the method of Lewis et al. (1986).
Cholecystokinin immunohistochemistry
The protocol for cholecystokinin (CCK) immunohistochemistry was similar to that used for PARV, but normal goat serum (NGS) was used instead of NHS, and after removing endogenous peroxidase activity, sections were incubated for 15 min in 1 % NaBH4 to increase the penetration of the primary antibody. We used rabbit-polyclonal antibody as the primary antibody, raised against CCK (1:10,000, #43842, Abcam, Cambridge, MA, USA) and as secondary antibody we used biotinylated anti-rabbit IgG (1:200, Vector # BA-1000, Vector Laboratories).
Calretinin immunohistochemistry
The protocol for calretinin (CR) immunohistochemistry was similar to that used for PARV, but NGS was used instead of NHS and after removing endogenous peroxidase activity, sections were incubated for 15 min in 1 % NaBH4. The primary antibody was rabbit-polyclonal antibody raised against CR (1:10,000, #7699/4, Swant) and the secondary antibody was biotinylated anti-rabbit IgG (1:200, Vector # BA-1000, Vector Laboratories).
Somatostatin immunohistochemistry
The protocol for somatostatin (SOM) immunohistochemistry was similar to that used for PARV, but NGS was used instead of NHS and the solution used to block non-specific binding included 10 % NGS, 0.5 % Triton X-100 (TX-100), and 0.1 % ovalbumin in 0.02 M KPBS. The primary antibody was rabbit-polyclonal antibody raised against SOM (1:5,000, a gift from G. Sperk, Univ. Innsbruck, Austria) and the primary antibody solution contained 0.1 % ovalbumin. The secondary antibody was biotinylated anti-rabbit IgG (1:200, Vector # BA-1000, Vector Laboratories).
Neuropeptide Y immunohistochemistry
The protocol for neuropeptide Y (NPY) immunohistochemistry was similar to that used for PARV, but NGS was used instead of NHS. The primary antibody was rabbit-polyclonal antibody raised against NPY (1:10,000, T-4070, Peninsula Laboratories, San Carlos, CA, USA) and the secondary antibody was biotinylated anti-rabbit IgG (1:200, Vector # BA-1000, Vector Laboratories).
Estimation of the total number of immunoreactive neurons in the hippocampus using unbiased stereology
For accurate laminar analysis, each immunopositive neuron throughout the entire septo-temporal axis of the hippocampus and dentate gyrus was plotted in a 1-in-16 series of 25 μm-thick sections (400 μm apart from each other) using the AccuStage MDPlot 5.3 graphical program and MD3 Microscope Digitizer (AccuStage, Shoreview, MN, USA) connected to a Leica DMRB microscope. We plotted all neuronal profiles that had at least 1 dendrite emanating from the soma. Reduction in axonal or dendritic immunolabeling was assessed qualitatively with light microscope.
The average number of immunopositive neurons plotted from one hippocampus of a control rat was 1,187 ± 35 (range 925–1,612) in PARV, 393 ± 12 (range 263–525) in CCK, 1,637 ± 35 (range 1,102–1,994) in CR, 1,650 ± 48 (range 1,160–2,419) in SOM, and 1,968 ± 35 (range 1,589–2,313) in NPY preparations. In our preliminary analysis (data not shown) used to define the counting parameters, CE was 1.4 % for PARV, 2.1 % for CCK, 0.9 % for CR, 1.2 % for SOM and 0.9 % for NPY-positive cells.
Next, the cytoarchitectonic boundaries of different hippocampal subfields were drawn on computer-generated plots from the immunostained and/or thionin-stained sections using camera lucida on a stereomicroscope equipped with a drawing tube. To calculate the total number of immunopositive cells, the following equation was used: N tot = ∑Q × 1/ssf × 1/asf × t/h, where ∑Q was the total number of neurons counted, the section sampling fraction (ssf) was 1/16, area sampling fraction (asf) was 1, and tissue sampling fraction (t/h, the height of the mounted section thickness divided by the dissector height) was 1 (West et al. 1991).
Control experiments
As reductions in the number of immunoreactive neurons may be associated with reduced expression of the epitope, we performed three control assays: (1) We estimated the total number of hilar neurons in different animal groups, (2) We demonstrated a localization of cell death marker, Fluoro-Jade B, in PARV-positive neurons, and (3) We demonstrated a reduction in substance P receptor (SPR) immunoreactivity in the dentate gyrus (a marker of PARV-positive neurons). As the most remarkable reduction of all interneuron classes after TBI was found in PARV-ir neurons, we focused on that particular interneuron type.
First, the total number of hilar neurons was estimated in Nissl-stained preparations using unbiased stereology as previously described by West et al. (1991). The analysis was performed using the Stereoinvestigator® software (MicroBrightField Inc., Williston, VT, USA) together with an Olympus BX50 microscope equipped with a motorized stage-controller and a Hitachi™ HVC20A camera. Every hippocampal section in a 1-in-8 series, yielding 12–13 sections per animal, was analyzed. A sampling grid of 120 × 120 μm was laid on the section. For every x–y step, cells were counted using a counting frame of 24 × 24 μm. Counting was performed throughout the section avoiding the neurons that were in focus at the surface of the section. Neuronal nuclei were counted only as they came into focus within each optical dissector. Glia, identified by size and cytological characteristics, were excluded from the counts. To calculate the total number of hilar cells, the following equation was used: N tot = ∑Q × 1/ssf × 1/asf × 1/tsf, where the section sampling fraction (ssf) was 1/8, area sampling fraction (asf, the area of counting frame divided by area of sampling grid) was 0.04, and tissue sampling fraction (tsf, the height of the mounted section thickness divided by the dissector height) was 1.
Next we assessed whether PARV-positive neurons known to contain lectin from wisteria floribunda (WFA) positive perineuronal nets co-label with Fluoro-Jade B, a marker of cell death, at early post-TBI time points (Härtig et al. 1992; Schmued and Hopkins 2000; Karetko-Sysa et al. 2011). TBI was induced and rats were perfused for histology at 1, 4, 24, or 48 h (2 at each time point) post-TBI as previously described. One series of free-floating sections was washed tree times in 0.02 M KPBS (pH 7.4). To remove endogenous peroxidase activity, sections were incubated in 1 % H2O2 in 0.02 M KPBS at RT for 15 min. After washing six times in 0.02 M KPBS, sections were incubated for 3 days (4 °C) in a primary antibody solution containing antibody raised against WFA (biotin conjugated, 1:4,000, L-1516, Sigma, Saint Louis, MO, USA) and 0.5 % TX-100 in 0.02 M KPBS. Sections were washed three times in 0.02 M KPBS and incubated in avidin–biotin (1:100, PK-4000, Vector Laboratories) in 0.02 M KPBS for 2 h at RT. After three washes in 0.02 M KPBS, the antibody was visualized with 0.1 % 3′,3′-diaminobenzidine (DAB, Pierce Chemicals) and 0.08 % H2O2 in 0.02 M KPBS. Sections were washed twice in KPBS, twice in 0.1 M PB, and mounted on gelatin-coated microscope slides. Slides were dried overnight at 37 °C. To perform Fluoro-Jade staining, WFA-stained sections were rinsed for 2 min in double-distilled H2O (ddH2O) and incubated for 20 min in 0.06 % KMnO4. After this, the slides were rinsed again for 2 min in ddH2O and incubated for 30 min in a solution containing 0.001 % Fluoro-Jade B, 0.1 % acetic acid in ddH2O. Slides were rinsed three times for 1 min in ddH2O and three times for 3 min in xylene. Finally, slides were coverslipped using DePeX® (BDH Chemical) as a mounting medium.
Third control assay was a qualitative analysis of substance P receptor (SPR) immunoreactivity, a marker of PARV-positive neurons (Sloviter et al. 2001, 2003). The protocol for SPR immunohistochemistry was similar to that used for PARV, but NGS was used instead of NHS, and after removing endogenous peroxidase activity, sections were incubated for 30 min in 0.05 M citrate buffer (pH 6.0) in 80 °C for better epitope retrieval. As primary antibody we used rabbit-polyclonal antibody raised against SPR (1:2,000, #AB5060, Chemicon, Temecula, CA, USA) and as secondary antibody we used biotinylated anti-rabbit IgG (1:200, Vector # BA-1000, Vector Laboratories).
Statistical analysis
Data were analyzed using IBM SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences in neuronal numbers between the groups were analyzed using the Kruskal–Wallis test. Post hoc analysis was performed using the Mann–Whitney U test. Interhemispheric differences were assessed by Wilcoxon’s test. Correlations were assessed using Spearman’s rho correlation coefficient. All data are expressed as mean ± SEM. A p value of less than 0.05 was considered statistically significant.
Results
Mortality
TBI Mortality within 24 h post-TBI was 23 % (10 of 44 rats) which indicates moderate severity of the TBI (McIntosh et al. 1989; Thompson et al. 2005).
SE Mortality within 24 h post-SE was 5 % (3 of 56 rats).
Epileptogenesis and epilepsy phenotype
PTZ test
Only the rats with TBI underwent the PTZ seizure susceptibility test, and the data are summarized in Table 1. Latency to the first epileptiform spike in rats with lateral FPI (n = 9) was shorter than that in controls (n = 6) (256 ± 53 s vs. 566 ± 150 s, p < 0.05). The number of EDs during the 60-min follow-up after PTZ injection was higher in injured animals as compared to that in controls (330 ± 84 vs. 109 ± 32, p < 0.05). The total number of spikes/60 min, latency to the first ED, or the mean duration of EDs did not differ between the injured and control rats.
PTZ administration resulted in behavioral signs of seizure activity in 75 % (12 of 16) of rats with TBI, which included head nodding, twitching, jerks, freezing, or generalized seizures. None of the sham-operated controls (n = 11) showed any behavioral seizures after PTZ injection.
Occurrence of spontaneous seizures at 6 months after epileptogenic insult
TBI None of the injured or control rats had spontaneous seizures during the 2-week continuous video-EEG monitoring performed at 6 months after lateral FPI or sham operation.
SE From the 28 stimulated rats, 11 (39 %) developed spontaneous seizures. Of these, 82 % (9/11) had a mean daily seizure frequency <1 seizure/day during the 2-week recording period. The average seizure duration in the “low seizure frequency group” was 106.9 ± 12.2 s. In the remaining 18 % of rats (2/11), the mean seizure frequency was ≥1 seizure/day with a mean seizure duration of 89.8 ± 12.5 s. The data are summarized in Table 2.
Histological analysis of different interneuron populations in the dentate gyrus and hippocampus proper: comparison of the two etiologies
Histologic analysis was done either at 1 month or 6 months post-insult. From the 1-month groups, we analyzed the following animals: (1) Rats with TBI (n = 9), (2) Controls for TBI (n = 8), (3) Rats with SE (n = 14), (4) Controls for SE (n = 10). From the 6-month groups, we selected animals from which we had successful 2-week video-EEG monitoring and high quality immunohistochemistry available. Consequently, we analyzed: (1) Rats with TBI (n = 9), (2) Sham-operated controls for TBI (n = 9), (3) Rats with SE and epilepsy (n = 7, 2 with “high” seizure frequency (≥1/day) and 5 with “low” (<1/day) seizure frequency), (4) Rats with SE and no epilepsy (n = 7), (5) Sham-operated controls for SE (n = 7).
PARV immunoreactivity
Stereological estimates of PARV-positive neurons in different subfields of the hippocampus proper and dentate gyrus are shown in Online Resource 1 (Supplementary Tables 1.1 and 1.2). Data are shown separately for the ipsilateral and contralateral sides.
Dentate gyrus
TBI 1-month group The number of PARV-ir neurons in the ipsilateral dentate gyrus was 62 % of that in controls (p < 0.001) (Fig. 2a; Supplementary Table 1.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells in the hilus (39 % of cells remaining as compared to controls, p < 0.001) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons did not differ from that in controls (Fig. 2a; Supplementary Table 1.1). Interhemispheric analysis revealed that the total number of PARV-ir cells was lower ipsilaterally than contralaterally (61 % of cells remaining, p < 0.01) (Fig. 2a; Supplementary Table 1.1). This was related to an ipsilateral reduction in cells both in the granule cell layer (68 % remaining as compared to the contralateral side, p < 0.01) and in the hilus (42 %, p < 0.01) (Supplementary Table 1.1).
Hippocampal interneurons innervating the perisomatic region of the principal cells. Bar graphs summarizing the total number of parvalbumin (PARV) and cholecystokinin (CCK) immunoreactive (ir) neurons in the dentate gyrus (upper panels), CA3 (middle panels), and CA1 (lower panels) in controls as well as at 1 or 6 months after TBI or SE. Ipsilateral (I) and contralateral (C) neuronal numbers are shown as percentages of corresponding control values (dashed line = 100 %). a In the dentate gyrus, TBI caused a progressive loss of PARV-ir (from 62 % at 1 month to 35 % at 6 months as compared to that in time-matched controls) and CCK-ir (from 90 to 63 %) neurons ipsilaterally as well as contralaterally (from 94 to 56 % and from 114 to 64 %, respectively). After SE, PARV-ir and CCK-ir neuronal numbers did not differ from those of controls. b In the CA3, TBI caused a progressive loss of PARV-ir both ipsilaterally (from 86 to 49 %) and contralaterally (from 100 to 57 %). After SE, the PARV-ir and CCK-ir neuronal numbers did not differ from those in controls. c Also in the CA1, TBI caused a progressive loss of PARV-ir both ipsilaterally (from 82 to 58 %) and contralaterally (from 99 to 67 %). After SE, the number of PARV-ir neurons was reduced ipsilaterally (80 %) at 6 months in rats with epilepsy. Statistical significances: *p < 0.05, **p < 0.01, ***p < 0.001 as compared to controls (Mann–Whitney U test); # p < 0.05, ## p < 0.01 as compared to the contralateral side (Wilcoxon); ¤ p < 0.05, ¤¤ p < 0.01, ¤¤¤ p < 0.001 as compared to the 1 month group (Mann–Whitney U test); ^ p < 0.05, ^^ p < 0.01, ^^^ p < 0.001 as compared to the TBI or SE group (Mann–Whitney U test); + p < 0.05 as compared to rats without epilepsy (Mann–Whitney U test)
Ipsilaterally, the immunohistochemical labeling of axons was remarkably decreased in the dentate gyrus, particularly in the supragranular region and the inner molecular layer of the infrapyramidal blade (data not shown). In addition, we found a patchy loss of immunopositive dendrites in the hilus and molecular layer (data not shown). Overall, ipsilaterally the reduction in immunoreactive elements was more pronounced septally than temporally (data not shown). Contralaterally, a reduction in the axonal immunolabeling was lighter than ipsilaterally (data not shown). Also, the immunopositive dendrites in the hilus and molecular layer were better preserved than ipsilaterally (data not shown).
TBI 6-month group Ipsilaterally, the total number of PARV-positive neurons in the whole dentate gyrus was 35 % of that in controls (p < 0.001) (Fig. 2a; Supplementary Table 1.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells in granule cell layer (36 % of cells remaining as compared to controls, p < 0.001) and the hilus (26 %, p < 0.001) (Supplementary Table 1.1). Contralaterally, the number of cells was 56 % of that in controls (p < 0.001) (Fig. 2a), which was related to a reduction in immunopositive neurons in the molecular layer (65 %, p < 0.05), granule cell layer (51 %, p < 0.01), and the hilus (60 %, p < 0.001) (Supplementary Table 1.1). Interhemispheric comparison revealed that the total number of PARV-ir cells ipsilaterally was 65 % of that contralaterally (p < 0.01) (Fig. 2a; Supplementary Table 1.1). This was related to a reduction in cells of the hilus (47 % of that of the contralateral side, p < 0.01) (Supplementary Table 1.1). In line with remarkable loss of immunopositive somata, we observed a robust decrease in the immunolabeling of perisomatic PARV containing axons in the granule cell layer (Fig. 3), which was more severe ipsilaterally than contralaterally (data not shown).
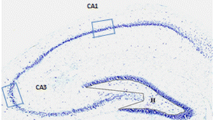
Parvalbumin (PARV) immunoreactivity (ir) in the ipsilateral hippocampus in the different animal groups. Panels on the left are from controls, panels in the middle are from rats with TBI 6 months earlier, and panels on the right are from rats with SE 6 months earlier (with epilepsy). a A computer-generated plot demonstrating the distribution of PARV-ir neurons in the septal hippocampus. Each dot represents one immunopositive neuron. A box with a dashed outline indicates an area from which the photomicrograph in panel c was taken. b PARV-ir neurons in the temporal hippocampus. Boxes with dashed outlines indicate regions from which the photomicrographs in panel d (b1) and e (b2) were taken. c A representative photomicrograph from the septal dentate gyrus (DG) in a control rat. The density of PARV-ir neurons (black arrows), axons (white arrows), and dendrites (thick black arrows) was high. d Distribution of PARV-ir elements in the temporal end of the dentate gyrus. e PARV-ir in the CA1. f A computer-generated plot showing the distribution of PARV-ir neurons in the septal hippocampus of a rat with TBI 6 months earlier. Note a remarkable loss on immunopositive neurons in all subfields and layers. g Note a slightly better preservation of PARV-positive neurons in the most temporal region of the hippocampus and in the dentate gyrus. h A photomicrograph taken at 6 months post-TBI reveals a robust decrease in immunopositive elements in the septal dentate gyrus. Very few immunopositive neurons (black arrow), axons (white arrow), or dendrites (thick black arrow) were remaining. Remarkable loss of immunopositive elements was found also in the i temporal end of the dentate gyrus. j In the CA1, the loss of PARV immunoreactivity was patchy. Note the loss of immunopositive cells and fibers particularly in the left half of the panel. On the right, some immunopositive cells (thin arrow) and dendrites (thick arrow) were still remaining. After SE (rat with epilepsy), neuronal density was largely comparable to that in controls both k septally and l temporally. Photomicrographs of immunostained sections from the m septal dentate gyrus, n temporal dentate gyrus, and o CA1 from an epileptic rat with SE 6 months earlier. Decrease of immunopositivity was seen only in the CA1. Scale bar equals 100 μm (all photomicrographs)
TBI: comparison of 1-month and 6-month group Ipsilaterally, the neuronal numbers in the whole dentate gyrus were lower at 6 months than at 1 month post-TBI (58 %, p < 0.001 as compared to the 1-month group) (Fig. 2a; Supplementary Table 1.1). The reduction was seen in all layers, including the molecular layer (65 % of cells remaining, p < 0.05), granule cell layer (52 %, p < 0.001), and the hilus (65 %, p < 0.01) (Supplementary Table 1.1). Contralaterally, the pattern was similar, with 55 % of PARV-ir neurons remaining as compared to 1 month post-TBI (p < 0.001) (Fig. 2a; Supplementary Table 1.1). This was related to a reduction in immunopositive cells of the molecular layer (57 % of cells remaining as compared to the 1-month group, p < 0.001), granule cell layer (50 %, p < 0.01), and the hilus (59 %, p < 0.001) (Supplementary Table 1.1).
Ipsilaterally, the reduction in axonal immunolabeling was much more robust at 6 months than at 1 month post-TBI (data not shown). Also, the reduction in the immunolabeling of PARV containing dendrites in the hilus and the molecular layer was more severe at 6 months than at 1 month post-TBI (data not shown). Contralaterally, the reduction in axonal immunolabeling was less severe than ipsilaterally but remarkably more pronounced at 6 months than at 1 month post-TBI (data not shown). Finally, the reduction in immunopositive elements at 1 month post-TBI was more limited to the septal end, whereas at 6 months post-TBI it extended throughout the entire septotemporal axis (data not shown). Contralaterally, the pattern was similar even though the reduction in immunoreactivity was milder (data not shown).
SE 1-month group The number of PARV-ir neurons in the whole dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2a; Supplementary Table 1.2). However, laminar analysis indicated a lower neuronal number in the granule cell layer ipsilaterally than contralaterally (81 % of cells remaining, p < 0.01) (Supplementary Table 1.2). Qualitative analysis did not reveal any change in axonal or dendritic immunolabeling (data not shown).
SE 6-month group The total number of PARV-positive neurons assessed in the whole SE group (including rats with and without epilepsy) did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2a; Supplementary Table 1.2). Ipsilaterally in the whole SE group, however, the total number of PARV-ir neurons in the dentate gyrus was 91 % of that contralaterally (p < 0.01) (Fig. 2a; Supplementary Table 1.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the hilus (88 %, p < 0.05) (Supplementary Table 1.2). In accord with a mild reduction in PARV-ir neuronal numbers, we observed a light reduction in the immunolabeling of PARV containing axons in the supragranular region and inner molecular layer ipsilaterally (Fig. 3).
When animals with or without epilepsy were compared, no differences were found in PARV-ir neuronal numbers (Fig. 2a; Supplementary Table 1.2) or axonal immunolabeling (data not shown). However, in rats with epilepsy, the number of PARV-ir neurons in the ipsilateral dentate gyrus was 87 % of that contralaterally (p < 0.05) (Fig. 2a; Supplementary Table 1.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the granule cell layer (87 % as compared to contralateral side, p < 0.05) (Supplementary Table 1.2). In rats without epilepsy, no differences in any of the cell counts were found when compared to controls or the contralateral side (Fig. 2a; Supplementary Table 1.2).
SE: comparison of 1-month and 6-month groups Ipsilaterally, the neuronal numbers in the whole group of animals did not differ (Fig. 2a; Supplementary Table 1.2). Contralaterally, however, the neuronal numbers in the 6-month group were lower in the entire dentate gyrus (87 % of cells remaining, p < 0.05) (Fig. 2a; Supplementary Table 1.2) as well as in the molecular layer (75 % of cells remaining, p < 0.01) and granule cell layer (86 % of cells remaining, p < 0.05) as compared to the 1-month group (Supplementary Table 1.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, the number of PARV-ir neurons in the dentate gyrus in rats with TBI 1 month earlier was 58 % of that in rats with SE 1 month earlier (p < 0.001) (Fig. 2a; Supplementary Table 1.1). Laminar analysis revealed that this was related to a reduction in immunopositive neurons of the granule cell layer (71 %, p < 0.001) and of the hilus (36 %, p < 0.001) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons in the dentate gyrus in the TBI group was 83 % of that in the SE group (p < 0.05) (Fig. 2a; Supplementary Table 1.1). This was related to a lower number of neurons in the molecular layer (81 % of that in SE group, p < 0.05) and in the granule cell layer (84 %, p < 0.01) (Supplementary Table 1.1).
The reduction in axonal immunolabeling was remarkably more pronounced at 1 month after TBI than at 1 month after SE (data not shown). Also, the reduction in the immunolabeling of PARV containing dendrites in the hilus and molecular layer was more severe at 1 month post-TBI than at 1 month post-SE (data not shown). Contralaterally, there was no difference in immunolabeling of PARV containing elements (data not shown).
Comparison of TBI and SE: 6-month groups Ipsilaterally, the number of PARV-ir neurons in the dentate gyrus of rats with TBI 6 months earlier was 37 % of that in rats with SE 6 months earlier (compared to the whole SE group, p < 0.001) (Fig. 2a; Supplementary Table 1.1). This was related to a lower number of neurons in granule cell layer (38 %, p < 0.001) and in the hilus (28 %, p < 0.001) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons in the TBI group was 52 % of that in the SE group (p < 0.001) (Fig. 2a; Supplementary Table 1.1). Laminar analysis revealed that this was related to a lower number of neurons in the molecular layer (61 %, p < 0.01), granule cell layer (49 %, p < 0.001), and hilus (53 %, p < 0.001) (Supplementary Table 1.1).
The reduction in axonal immunolabeling was remarkably more pronounced at 6 months post-TBI than at 6 months post-SE (Fig. 3). Also, the loss of dendritic labeling was more severe in the TBI group than the SE group (Fig. 3). In the TBI group, there were very few immunopositive dendritic elements remaining in the dentate gyrus, whereas in the SE group the loss of dendrites was patchy (Fig. 3). In addition, the reduction of immunopositive elements at 6 months post-TBI extended more widely throughout the entire septotemporal axis as compared to that in the SE group (Fig. 3). Contralaterally, the pattern was similar even though the difference between the groups was not as evident as that seen ipsilaterally (data not shown).
CA3 subfield of the hippocampus proper
TBI 1-month group The number of PARV-ir neurons in the whole CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 1.1). Interhemispheric analysis revealed, however, that ipsilaterally the number of PARV-ir neurons in the whole CA3 was 78 % of that on the contralateral side (p < 0.05) (Fig. 2b; Supplementary Table 1.1). The immunolabeling of the perisomatic terminal plexus was decreased in the ipsilateral CA3 pyramidal cell layer, particularly in the CA3a region (data not shown). In addition, rats had a remarkable loss of immunopositive dendrites, which was most pronounced in the CA3a stratum radiatum (data not shown).
TBI 6-month group Ipsilaterally, the number of PARV-ir neurons was only 49 % of that in controls (p < 0.01) (Fig. 2b; Supplementary Table 1.1) and the decrease involved all layers of the CA3 (Supplementary Table 1.1). The number of neurons in the stratum oriens was 41 % (p < 0.001) in the pyramidal cell layer 49 % (p < 0.01), and in the stratum radiatum it was 58 % (p < 0.05) of that in controls (Supplementary Table 1.1). Contralaterally, we also found a decreased number of cells (57 % of cells remaining as compared to controls, p < 0.01) (Fig. 2b; Supplementary Table 1.1). Laminar analysis revealed that this was related to a reduction in PARV-ir neurons of stratum oriens (58 %, p < 0.05) and pyramidal cell layer (55 %, p < 0.05) (Supplementary Table 1.1). We did not find any interhemispheric differences (Fig. 2b; Supplementary Table 1.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of PARV-ir neurons at 6 months was 50 % of that at 1 month post-TBI (p < 0.05) (Fig. 2b; Supplementary Table 1.1). The reduction was found both in the stratum oriens (32 %, p < 0.001) and stratum radiatum (54 %, p < 0.05) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons in the whole CA3 at 6 months was 44 % of that at 1 month post-TBI (p < 0.01) (Fig. 2b; Supplementary Table 1.1). Like ipsilaterally, the decrease was also present contralaterally in both the stratum oriens (29 %, p < 0.001) and stratum radiatum (51 %, p < 0.05) (Supplementary Table 1.1).
Ipsilaterally, the immunolabeling of PARV containing axonal plexus was remarkably reduced in the pyramidal cell layer (data not shown). The dendritic loss was most pronounced in the stratum radiatum of the CA3a (data not shown). Also contralaterally, we found a reduction in the immunolabeling of the axonal plexus in the CA3a, even though milder than that ipsilaterally (data not shown). Also, the loss of immunopositive dendrites was most pronounced in the CA3a stratum radiatum (data not shown). Importantly, there was a remarkable inter-animal variability in PARV-ir in the CA3, varying from being an almost unnoticeable unilateral reduction to a remarkable bilateral decrease in immunoreactivity (data not shown).
SE 1-month group The number of PARV-ir neurons in the whole CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 1.2). Also, we did not find any interhemispheric differences (Fig. 2b; Supplementary Table 1.2). Qualitative analysis did not reveal any change in axonal or dendritic immunolabeling (data not shown).
SE 6-month group At 6 months post-SE, the number of PARV-ir neurons in the whole CA3 as assessed in the entire SE group (epileptic and non-epileptic animals) did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 1.2). Ipsilaterally, however, the number of PARV-ir neurons in the whole CA3 was 87 % of that contralaterally (p < 0.05) (Fig. 2b; Supplementary Table 1.2). Laminar analysis revealed that this was related to a reduction in PARV-ir neurons of the pyramidal cell layer (87 % of that on contralateral side, p < 0.05) (Supplementary Table 1.2). The distribution of immunopositive axons showed occasional mild patchiness in the perisomatic region of the pyramidal cell layer in cases with reduced numbers of immunopositive neurons (data not shown). The patchy reduction of immunopositive dendrites was visible, even though mild, in the stratum oriens and stratum radiatum in cases with reduced neuronal numbers (data not shown).
Ipsilaterally, in a subgroup of animals with epilepsy, the number of PARV-ir neurons in the whole CA3 was lower than that in rats without seizures (76 %, p < 0.05) (Fig. 2b; Supplementary Table 1.2). In particular, the number of PARV-ir neurons was reduced in the pyramidal cell layer (71 % of that in rats without seizures, p < 0.01) (Supplementary Table 1.2). Interhemispheric analysis revealed that in epileptic rats, the number of PARV-ir neurons in the ipsilateral whole CA3 was 77 % of that on the contralateral side (p < 0.05) (Fig. 2b; Supplementary Table 1.2). The reduction of cells was most prominent in the pyramidal cell layer (75 % remaining as compared to contralateral side, p < 0.05) (Supplementary Table 1.2). Like in the dentate gyrus, in non-epileptic rats, the neuronal counts did not differ from those in controls (Fig. 2b; Supplementary Table 1.2). Also, no interhemispheric differences were observed (Fig. 2b; Supplementary Table 1.2). In addition, no difference was found in the distribution of immunopositive axons and dendrites between epileptic and non-epileptic animals (data not shown).
SE: comparison of 1-month and 6-month groups Ipsilaterally, the number of PARV-ir neurons in the whole CA3 at 6 months was 74 % of that at 1 month post-SE (p < 0.05) (Fig. 2b; Supplementary Table 1.2). This was related to reduced neuronal numbers in the stratum oriens (70 %, p < 0.01) and pyramidal cell layer (75 %, p < 0.05) (Supplementary Table 1.2). Contralaterally, we did not find any differences (Fig. 2b; Supplementary Table 1.2). At 6 months post-SE, the distribution of immunopositive elements showed occasional mild patchiness, which was not seen at 1 month post-SE (data not shown).
Comparison of TBI and SE: 1-month groups Ipsilaterally, the number of immunopositive neurons at 1 month post-TBI was 75 % of that in rats with SE 1 month earlier (p < 0.05) (Fig. 2b; Supplementary Table 1.1). Laminar analysis revealed that this was related to a lower number of neurons in the pyramidal cell layer (72 % of that at 1 month post-SE, p < 0.01) (Supplementary Table 1.1). Contralaterally, we did not find any differences (Fig. 2b; Supplementary Table 1.1). The reduction in immunolabeling of PARV containing elements was more pronounced ipsilaterally after TBI than SE (data not shown).
Comparison of TBI and SE: 6-month groups Ipsilaterally, the number of PARV-ir neurons at 6 months post-TBI was 50 % of that at 6 months post-SE (p < 0.01) (Fig. 2b; Supplementary Table 1.1). The difference was seen in all layers of the CA3, including the stratum oriens (37 %, p < 0.001), pyramidal cell layer (55 %, p < 0.01), and stratum radiatum (53 %, p < 0.01) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons in the whole CA3 at 6 months post-TBI was 49 % of that in the SE group (p < 0.001) (Fig. 2b; Supplementary Table 1.1). Laminar analysis revealed that this was related to a reduction of PARV-ir neurons in the stratum oriens (41 % of that at 6 months post-SE, p < 0.01), pyramidal cell layer (52 %, p < 0.001), and stratum radiatum (54 %, p < 0.01) (Supplementary Table 1.1).
Like in the dentate gyrus, the loss of PARV-ir axons and dendrites was remarkably more severe and widespread after TBI than SE (data not shown).
CA1 subfield of the hippocampus proper
TBI 1-month group Ipsilaterally, the number of PARV-ir neurons in the whole CA1 was 82 % of that in controls (p < 0.05) (Fig. 2c; Supplementary Table 1.1). Particularly, the decrease was seen in the pyramidal cell layer where 80 % of cells were remaining (p < 0.01 as compared to controls) (Supplementary Table 1.1). Contralaterally, no differences were found as compared to controls (Fig. 2c; Supplementary Table 1.1). Consequently, at 1 month post-TBI, the number of PARV-ir neurons in the whole ipsilateral CA1 was 75 % of that contralaterally (p < 0.01) (Fig. 2c; Supplementary Table 1.1). Laminar analysis revealed that this was related to a reduction in PARV-ir neurons of the stratum oriens (79 % of that of the contralateral side, p < 0.05) and the pyramidal cell layer (74 %, p < 0.05) (Supplementary Table 1.1). Ipsilaterally, the distribution of perisomatic immunoreactivity was decreased in the pyramidal cell layer (data not shown). We also found a remarkable loss of immunopositive dendrites in the proximal stratum oriens and stratum radiatum as compared to controls (data not shown).
TBI 6-month group Ipsilaterally, the number of PARV-ir neurons in the whole CA1 was 58 % of that in controls (p < 0.01) (Fig. 2c; Supplementary Table 1.1). Remarkable decreases were seen in the all layers of the CA1. The number of cells in the stratum oriens was 59 % (p < 0.01), pyramidal cell layer 60 % (p < 0.01) and stratum radiatum 48 % (p < 0.01) of that in controls (Supplementary Table 1.1). Contralaterally, the pattern of reduction in immunoreactivity was similar but milder. The number of PARV-ir neurons in the whole contralateral CA1 was 67 % of that in controls (p < 0.01) (Fig. 2c; Supplementary Table 1.1). This was related to a reduction in neuronal numbers within the stratum oriens (74 %, p < 0.01), pyramidal cell layer (64 %, p < 0.01) and stratum radiatum (50 %, p < 0.001) (Supplementary Table 1.1). We did not find any interhemispheric differences in neuronal numbers (Fig. 2c; Supplementary Table 1.1).
Ipsilaterally, the immunolabeling of PARV containing axons was remarkably reduced in the pyramidal cell layer (Fig. 3). The dendritic loss was most pronounced in the proximal stratum oriens and stratum radiatum (data not shown). Contralaterally, we also found a reduction in the immunolabeling of the perisomatic axonal plexus, even though milder than that seen ipsilaterally (data not shown). The loss of immunoreactive dendrites was visible, but milder than ipsilaterally, and most pronounced in the proximal CA1 (data not shown). Like in the CA3, there was a remarkable inter-animal variability in PARV-ir in the CA1 (data not shown).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of PARV-ir neurons in the CA1 at 6 months was 74 % of that at 1 month post-TBI (p < 0.05) (Fig. 2c; Supplementary Table 1.1). This was related to a reduction in immunopositive cells of the stratum oriens (71 %, p < 0.01) and stratum radiatum (52 %, p < 0.01) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons was 63 % of that at 1 month post-TBI (p < 0.001) (Fig. 2c; Supplementary Table 1.1). The number of cells in the stratum oriens was 66 % (p < 0.01), pyramidal cell layer 65 % (p < 0.01) and stratum radiatum 41 % (p < 0.001) of that measured at 1 month after TBI (Supplementary Table 1.1).
Ipsilaterally, at 1 month post-TBI, the reduction of immunopositive elements was typically limited to the proximal CA1, whereas at 6 month post-TBI the reduction in the immunolabeling of the axonal plexus was more widespread (data not shown). Also contralaterally, the reduction in axonal immunolabeling was remarkably more robust at 6 months post-TBI as compared to the 1-month group (data not shown).
SE 1-month group The number of PARV-ir neurons in the CA1 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2c; Supplementary Table 1.2). Also, we did not find any interhemispheric differences (p > 0.05) (Fig. 2c; Supplementary Table 1.2).
SE 6-month group The number of PARV-ir neurons in the entire CA1 as assessed in the whole SE group did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2c; Supplementary Table 1.2). Ipsilaterally, however, laminar analysis revealed a reduction in PARV-ir neurons in the pyramidal cell layer (81 % of cells remaining as compared to controls, p < 0.05) (Supplementary Table 1.2). Like in the CA3, the distribution of immunopositive axons showed occasional mild patchiness in the perisomatic region of the pyramidal cell layer in cases with reduced numbers of immunopositive neurons (Fig. 3). We also found a patchy reduction in the immunolabeling of PARV containing dendrites in the stratum oriens and stratum radiatum in cases with a reduced number of immunopositive somata (Fig. 3).
Ipsilaterally, in a subgroup of rats with epilepsy, the number of PARV-ir neurons in the whole CA1 was 80 % of that in controls (p < 0.01) (Fig. 2c; Supplementary Table 1.2). Particularly, a decrease was seen in the pyramidal cell layer (70 %, p < 0.01) (Supplementary Table 1.2). Ipsilaterally, when animals with or without epilepsy were compared, the total number of PARV-ir neurons in the CA1 in rats with epilepsy was 81 % of that in rats without epilepsy (p < 0.05) (Fig. 2c; Supplementary Table 1.2). In particular, epileptic animals had reduced numbers of PARV-ir neurons in the stratum oriens (83 %, p < 0.05) and pyramidal cell layer (75 %, p < 0.05) (Supplementary Table 1.2). Contralaterally, the number of PARV-ir neurons in the pyramidal cell layer in epileptic rats was 63 % of that in non-epileptic animals (p < 0.05) (Supplementary Table 1.2). There were no interhemispheric differences in any of the different animal groups (Fig. 2c; Supplementary Table 1.2). In addition, no difference was found in the distribution of immunopositive processes between the epileptic and non-epileptic animals (data not shown).
SE: comparison of 1-month and 6-month groups The number of PARV-ir neurons at 6 months post-SE in the entire CA1 as assessed in the whole SE group did not differ from that at 1 month post-SE (Fig. 2c; Supplementary Table 1.2). Ipsilaterally, the total number of PARV-ir neurons in the CA1 at 6 months post-SE in the pyramidal cell layer was 84 % (p < 0.05) and in the stratum radiatum it was 48 % (p < 0.001) of that at 1 month post-SE (Supplementary Table 1.2). Contralaterally, the number of immunopositive cells in the stratum radiatum at 6 months post-SE was 55 % (p < 0.001) of that at 1 month post-SE (Supplementary Table 1.2). Unexpectedly, the 1 month group had a lower number of PARV-ir neurons in the stratum oriens that the 6 months group (76 %, p < 0.05) (Supplementary Table 1.2). Like in the CA3, at 6 months post-SE the distribution of immunopositive processes showed occasional mild patchiness, which was not seen at 1 month post-SE (data not shown).
Comparison of TBI and SE: 1-month groups Ipsilaterally, in rats with TBI, the number of PARV-ir neurons in the CA1 was 78 % of that in rats with SE 1 month earlier (p < 0.01) (Fig. 2c; Supplementary Table 1.1). Laminar analysis revealed that this was related to a lower number of neurons in the pyramidal cell layer (75 %, p < 0.01) and stratum radiatum (65 %, p < 0.01) (Supplementary Table 1.1). Contralaterally, we did not find any differences (Fig. 2c; Supplementary Table 1.1). The reduction in PARV-ir processes was more pronounced after TBI than after SE (data not shown).
Comparison of TBI and SE: 6-month groups Ipsilaterally, in rats with TBI, the number of PARV-ir neurons in the CA1 was 61 % of that in the whole group of rats with SE 6 months earlier (p < 0.001) (Fig. 2c; Supplementary Table 1.1). Particularly, a reduction was seen in the stratum oriens (52 %, p < 0.001) and pyramidal cell layer (71 %, p < 0.05) (Supplementary Table 1.1). Contralaterally, the number of PARV-ir neurons in the TBI group was 65 % of that in the SE group (p < 0.01) (Fig. 2c; Supplementary Table 1.1). Laminar analysis revealed that this was related to a lower number of neurons in the stratum oriens (58 %, p < 0.001) (Supplementary Table 1.1). Both ipsilaterally (Fig. 3) and contralaterally (data not shown), the reduction of immunopositive processes was remarkably more pronounced after TBI than after SE.
CCK immunoreactivity
Stereological estimates of CCK-positive neurons in different subfields of the hippocampus proper and dentate gyrus are shown in Online Resource 2 (Supplementary Tables 2.1. and 2.2). Data are shown separately for the ipsilateral and contralateral sides.
Dentate gyrus
TBI 1-month group The number of CCK-ir neurons in the whole dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2a; Supplementary Table 2.1). However, interhemispheric analysis revealed that the total number of CCK-ir cells was lower ipsilaterally than contralaterally (75 % of cells remaining, p < 0.05) (Fig. 2a; Supplementary Table 2.1). This was related to a reduction in CCK-ir neurons in the hilus (69 % remaining as compared to the contralateral side, p < 0.01) (Supplementary Table 2.1).
TBI 6-month group Ipsilaterally, the total number of CCK-ir neurons in the whole dentate gyrus was 63 % of that in controls (p < 0.05) (Fig. 2a; Supplementary Table 2.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the hilus (56 % of cells remaining as compared to controls, p < 0.01) (Supplementary Table 2.1). Contralaterally, the number of CCK-ir cells in the dentate gyrus was 64 % of that in controls, (p < 0.01) (Fig. 2a; Supplementary Table 2.1) which was related to a reduction in immunopositive neurons within the hilus (57 %, p < 0.01) (Supplementary Table 2.1). We did not find any interhemispheric differences (Fig. 2a; Supplementary Table 2.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of CCK-ir neurons at 6 months post-TBI was 65 % of that at 1 month post-TBI (p < 0.05) (Fig. 2a; Supplementary Table 2.1). The reduction was found in the hilus (67 %, p < 0.05) (Supplementary Table 2.1). Contralaterally, the number of CCK-ir neurons at 6 months was 54 % of that at 1 month post-TBI (p < 0.05) (Fig. 2a; Supplementary Table 2.1). Like ipsilaterally, the decrease was also present in the hilus contralaterally (49 %, p < 0.01) (Supplementary Table 2.1).
SE 1-month group The number of CCK-ir neurons in the whole dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2a; Supplementary Table 2.2). Also, we did not find any interhemispheric differences (Fig. 2a; Supplementary Table 2.2).
SE 6-month group The number of CCK-ir neurons assessed in the whole SE group (including rats with and without epilepsy) did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2a; Supplementary Table 2.2). Also, we did not find any interhemispheric differences (Fig. 2a; Supplementary Table 2.2).
Ipsilaterally, in a subgroup of rats with epilepsy, the number of CCK-ir neurons in the granule cell layer was 79 % of that contralaterally (p < 0.05) (Supplementary Table 2.2). In rats without epilepsy, no differences in any of the cell counts were found when compared to controls or the contralateral side (Fig. 2a; Supplementary Table 2.2).
SE: comparison of the 1-month and 6-month groups Ipsilaterally, we did not find any differences between groups (Fig. 2a; Supplementary Table 2.2). Contralaterally, the number of immunopositive cells in granule cell layer at 6 months post-SE was 73 % (p < 0.05) of that at 1 month post-SE (Supplementary Table 2.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, in rats with TBI 1 month earlier, the number of CCK-ir neurons in the hilus was 78 % of that in rats with SE 1 month earlier (p < 0.05) (Supplementary Table 2.1). Contralaterally, we did not find any differences (Fig. 2a; Supplementary Table 2.1).
Comparison of TBI and SE: 6-month groups Ipsilaterally, in rats with TBI 6 months earlier, the number of CCK-ir neurons in the dentate gyrus was 51 % of that in the whole group of rats with SE 6 months earlier (p < 0.001) (Fig. 2a; Supplementary Table 2.1). Particularly, a reduction was seen within the hilus (45 %, p < 0.001) (Supplementary Table 2.1). Contralaterally, the number of CCK-ir neurons in the TBI group was 61 % of that in the SE group (p < 0.01) (Fig. 2a; Supplementary Table 2.1). Laminar analysis revealed that this was also related to a lower number of immunopositive neurons in the hilus (52 %, p < 0.001) (Supplementary Table 2.1).
Immunopositive axons Overall, the pericellular immunopositivity around the granule cells (particularly the upper third and supragranular region) in the dentate gyrus was substantial despite loss of CCK-positive neurons in the hilus (Fig. 4). Instead, the number of pericellular plexi around the presumed hilar mossy cells was reduced in regions with loss of immunopositive hilar cells, particularly in the infrapyramidal blade (Fig. 4).
Cholecystokinin (CCK) immunoreactivity (ir) in the ipsilateral hippocampus in the different animal groups. Panels on the left are from controls, panels in the middle from rats with TBI 6 months earlier, and panels on the right from rats with SE 6 months earlier (with epilepsy). a A computer-generated plot demonstrating the distribution of CCK-positive neurons in the septal hippocampus. Each dot represents one immunopositive neuron. A box with a dashed outline indicates an area from which the photomicrograph in panel c was taken. b CCK-positive neurons in the temporal hippocampus. c A representative photomicrograph from the septal dentate gyrus (DG) in controls. Arrows point to immunopositive neurons. d Computer-generated plot showing the distribution of CCK-positive neurons in the septal hippocampus of a rat with TBI 6 months earlier. Note a loss of immunopositive neurons mainly in the dentate gyrus. e Distribution of immunopositive cells in the temporal hippocampus. f A photomicrograph taken from an immunostained section sampled at 6 months post-TBI. Only a few immunopositive neurons (arrows) were remaining mainly at the border between the hilus and suprapyramidal blade of the granule cell layer. After SE (rat with epilepsy), neuronal density was comparable to that in controls both g septally and h temporally. i A photomicrograph from an immunostained section of the septal dentate gyrus. The density of immunopositive neurons (arrows) is comparable to that in controls (c). Scale bar equals 100 μm (all photomicrographs)
CA3 subfield of the hippocampus proper
TBI 1-month group The number of CCK-ir neurons in the whole CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 2.1). Also, we did not find any interhemispheric differences (Fig. 2b; Supplementary Table 2.1).
TBI 6-month group The number of CCK-ir neurons in the whole CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 2.1). Also, we did not find any interhemispheric differences (Fig. 2b; Supplementary Table 2.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of CCK-ir neurons in the CA3 did not differ between time points (Fig. 2b; Supplementary Table 2.1). Contralaterally, the number of cells in the whole CA3 at 6 months post-TBI was 64 % of that in 1 month post-TBI, (p < 0.01) (Fig. 2b; Supplementary Table 2.1) which was related to a reduction in immunopositive neurons in the stratum oriens (50 %, p < 0.05), pyramidal cell layer (63 %, p < 0.05), and stratum radiatum (69 %, p < 0.01) (Supplementary Table 2.1).
SE 1-month group The number of CCK-ir neurons in the whole CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 2.2). Also, we did not find any interhemispheric differences (Fig. 2b; Supplementary Table 2.2).
SE 6-month group The number of CCK-ir neurons in the whole CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 2.2). Also, we did not find any interhemispheric differences (Fig. 2b; Supplementary Table 2.2).
SE: comparison of 1-month and 6-month groups The number of CCK-ir neurons in the CA3 did not differ between time points either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 2.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, the number of CCK-ir neurons in the CA3 did not differ between groups (Fig. 2b; Supplementary Table 2.1; Supplementary Table 2.2). Contralaterally, the number of cells in the whole CA3 at 1 month post-SE was 78 % of that at 1 month post-TBI, (p < 0.01) (Fig. 2b; Supplementary Table 2.2) which was related to a lower number of immunopositive neurons in the stratum oriens (58 %, p < 0.05) (Supplementary Table 2.2).
Comparison of TBI and SE: 6-month groups The number of CCK-ir neurons in the CA3 did not differ between groups either ipsilaterally or contralaterally (Fig. 2b; Supplementary Table 2.1; Supplementary Table 2.2).
Immunopositive axons In line with only a mild loss of CCK-ir cells in the CA3 in different experimental conditions, we found substantial pericellular axonal immunoreactivity around the CA3 pyramidal cells (data not shown).
CA1 subfield of the hippocampus proper
TBI 1-month group The number of CCK-ir neurons in the whole CA1 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2c; Supplementary Table 2.1). Also, we did not find any interhemispheric differences (Fig. 2c; Supplementary Table 2.1).
TBI 6-month group Ipsilaterally, the number of CCK-positive neurons in the stratum oriens was 63 % (p < 0.01) and in pyramidal cell layer it was 61 % (p < 0.05) of that in controls (Supplementary Table 2.1). Contralaterally, we did not find any differences (Fig. 2c; Supplementary Table 2.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of CCK-ir neurons in the whole CA1 was lower at 6 months than at 1 month post-TBI (64 %, p < 0.01) (Fig. 2c; Supplementary Table 2.1). The reduction was seen in all layers, including the stratum oriens (42 % of cells remaining, p < 0.001), pyramidal cell layer (59 %, p < 0.05), and stratum radiatum (74 %, p < 0.05) (Supplementary Table 2.1). Contralaterally, the pattern was similar, with 69 % of CCK-ir neurons remaining as compared to 1 month post-TBI (p < 0.01) (Fig. 2c; Supplementary Table 2.1). This was related to a reduction in immunopositive cells within the stratum oriens (59 % of cells remaining as compared to the 1 month group, p < 0.01), pyramidal cell layer (67 %, p < 0.05), and stratum radiatum (72 %, p < 0.05) (Supplementary Table 2.1).
SE 1-month group The number of CCK-ir neurons in the whole CA1 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2c; Supplementary Table 2.2). Also, we did not find any interhemispheric differences (Fig. 2c; Supplementary Table 2.2).
SE 6-month group The number of CCK-ir neurons in the whole CA1 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 2c; Supplementary Table 2.2). Also, we did not find any interhemispheric differences (Fig. 2c; Supplementary Table 2.2).
SE: comparison of the 1-month and 6-month groups Ipsilaterally, the number of CCK-ir neurons in the CA1 did not differ between time points (Fig. 2c; Supplementary Table 2.2). Contralaterally, at 6 months post-SE the number of CCK-ir neurons in the stratum oriens was 72 %, and in the stratum radiatum 78 % of that at 1 month post-SE (p < 0.05) (Supplementary Table 2.2). Unlike expected, the number of CCK-ir neurons at 1 month post-SE was 70 % of that at 6 months post-SE in the pyramidal layer (p < 0.01) (Supplementary Table 2.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, the number of CCK-ir neurons at 1 month post-SE in the stratum oriens was 72 % (p < 0.05), and in the pyramidal cell layer 74 % (p < 0.05) of that at 1 month post-TBI (Supplementary Table 2.2). Contralaterally, the neuronal numbers in the whole CA1 were lower at 1 month post-SE than at 1 month post-TBI (86 %, p < 0.05) (Fig. 2c; Supplementary Table 2.2). The reduction was seen in the pyramidal cell layer (68 % of cells remaining, p < 0.05) (Supplementary Table 2.2).
Comparison of TBI and SE: 6-month groups Ipsilaterally, the number of CCK-ir neurons in the CA1 did not differ between groups (Fig. 2c; Supplementary Table 2.1; Supplementary Table 2.2). Contralaterally, the number of CCK-positive neurons at 6 months post-TBI in the pyramidal cell layer was 69 % (p < 0.05) of that at 6 months post-SE (Supplementary Table 2.1).
Immunopositive axons Like in the CA3, we found substantial pericellular axonal immunoreactivity around CA1 pyramidal cells (data not shown).
CR immunoreactivity
Stereological estimates of CR-positive neurons in different subfields of the hippocampus proper and dentate gyrus are shown in Online Resource 3 (Supplementary Tables 3.1 and 3.2). Data are shown separately for the ipsilateral and contralateral sides.
Dentate gyrus
TBI 1-month group The number of CR-ir neurons in the ipsilateral dentate gyrus was 77 % of that in controls (p < 0.05) (Fig. 5a; Supplementary Table 3.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the granule cell layer (72 % of cells remaining as compared to controls, p < 0.01) and in the hilus (75 %, p < 0.05) (Supplementary Table 3.1). Contralaterally, the number of CR-ir neurons did not differ from that in controls (Fig. 5a; Supplementary Table 3.1). Interhemispheric analysis revealed that the total number of CR-ir cells was lower ipsilaterally than contralaterally (77 %, p < 0.05) (Fig. 5a; Supplementary Table 3.1). This was related to an ipsilateral reduction in cells both in the granule cell layer (78 % remaining as compared to the contralateral side, p < 0.05) and in the hilus (71 %, p < 0.01) (Supplementary Table 3.1).
Hippocampal interneurons innervating the principal cell dendrites. Bar graphs summarizing the total number of calretinin (CR), somatostatin (SOM) and neuropeptide Y (NPY) immunoreactive (ir) neurons in the dentate gyrus (upper panels), CA3 (middle panels), and CA1 (lower panels) in controls as well as at 1 or 6 months after TBI or SE. Ipsilateral (I) and contralateral (C) neuronal numbers are shown as percentages of the corresponding control values (dashed line = 100 %). a In the dentate gyrus, the number of CR-ir neurons was reduced ipsilaterally (to 77 % of that in controls) at 1 month post-TBI and both ipsilaterally (74 %) and contralaterally (77 %) at 6 months post-TBI. The number of SOM-ir neurons was reduced ipsilaterally at 1 month (46 %) and at 6 month post-TBI (55 %). TBI caused a progressive loss of NPY-ir neurons ipsilaterally between 1 (59 %) and 6 (51 %) months. After SE, we found a loss of NPY-ir neurons in the dentate gyrus both ipsilaterally (33 %) and contralaterally (37 %) in a subgroup of rats with epilepsy at 6 months post-SE. b In the CA3, CR-ir, SOM-ir or NPY-ir neuronal numbers did not differ from those in controls at 1 month post-TBI. At 6 months post-TBI, however, there was a reduction in the number of CR-ir neurons contralaterally (81 %), SOM-ir neurons ipsilaterally (64 %), and NPY-ir neurons ipsilaterally (76 %). Similarly, at 1 month post-SE, the neuronal numbers did not differ from those in controls. At 6 months post-SE, in the whole group of animals, CR-ir neurons remained unchanged but there was a reduction in SOM-ir neurons contralaterally (78 %) and in NPY-ir neurons ipsilaterally (75 %). Interestingly, in rats with epilepsy, the number of NPY-ir neurons was reduced both ipsilaterally (54 %) and contralaterally (57 %). c In the CA1, CR-ir, SOM-ir or NPY-ir neuronal numbers did not differ from those in controls at 1 month post-TBI. At 6 months post-TBI, however, the number of SOM-ir neurons was reduced both ipsilaterally (75 %) and contralaterally (78 %). After SE, we found a reduction in the number of CR-ir and SOM-ir neurons contralaterally (89 and 86 %, respectively) at 1 month post-SE, which were not, however, significant at 6 months post-SE. Statistical significances: *p < 0.05, **p < 0.01, ***p < 0.001 as compared to controls (Mann–Whitney U test); # p < 0.05, ## p < 0.01 as compared to the contralateral side (Wilcoxon); ¤ p < 0.05, ¤¤ p < 0.01, ¤¤¤ p < 0.001 as compared to the 1 month group (Mann–Whitney U test); ^ p < 0.05, ^^ p < 0.01, ^^^ p < 0.001 as compared to the TBI or SE group (Mann–Whitney U test); + p < 0.05 as compared to rats without epilepsy (Mann–Whitney U test)
TBI 6-month group Ipsilaterally, the total number of CR-ir neurons in the dentate gyrus was 74 % of that in controls (p < 0.001) (Fig. 5a; Supplementary Table 3.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the hilus (66 % of cells remaining as compared to controls, p < 0.001) (Supplementary Table 3.1). Contralaterally, the number of CR-ir cells in the dentate gyrus was 77 % of that in controls, (p < 0.05) (Fig. 5a; Supplementary Table 3.1) which was related to fewer immunopositive neurons in the granule cell layer (79 %, p < 0.05) (Supplementary Table 3.1). Interhemispheric analysis revealed that the total number of CR-ir cells was lower ipsilaterally than contralaterally (86 % of cells remaining, p < 0.05) (Fig. 5a; Supplementary Table 3.1). This was related to an ipsilateral reduction in cells within the hilus (76 % remaining as compared to the contralateral side, p < 0.05) (Supplementary Table 3.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of CR-ir neurons did not differ between groups (Fig. 5a; Supplementary Table 3.1). Contralaterally, in the dentate gyrus, the number of CR-ir neurons at 6 months was 86 % of that at 1 month post-TBI (p < 0.05) (Fig. 5a; Supplementary Table 3.1).
SE 1-month group The number of CR-ir neurons in the dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5a; Supplementary Table 3.2). Also, we did not find any interhemispheric differences (Fig. 5a; Supplementary Table 3.2).
SE 6-month group The number of CR-ir neurons assessed in the whole SE group (including rats with and without epilepsy) did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5a; Supplementary Table 3.2). Also, we did not find any interhemispheric differences (Fig. 5a; Supplementary Table 3.2).
Ipsilaterally, in a subgroup of rats with epilepsy, the number of CR-ir neurons in the molecular layer was 76 % of that in a subgroup of rats without epilepsy (p < 0.05) (Supplementary Table 3.2). Also contralaterally, the number of CR-ir neurons in the molecular layer was decreased when rats with epilepsy were compared to rats without epilepsy (76 %, p < 0.05) (Supplementary Table 3.2). In rats without epilepsy, no differences in any of the cell counts were found when compared to controls or the contralateral side (Fig. 5a; Supplementary Table 3.2).
SE: comparison of 1-month and 6-month groups Ipsilaterally, we did not find any differences between groups (Fig. 5a; Supplementary Table 3.2). Contralaterally, the number of CR-ir cells in the molecular layer at 6 months post-SE was 76 % (p < 0.01) of that at 1 month post-SE (Fig. 5a; Supplementary Table 3.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, at 1 month post-TBI, the number of CR-ir neurons in the dentate gyrus was 73 % of that at 1 month post-SE (p < 0.001) (Fig. 5a; Supplementary Table 3.1). Laminar analysis revealed that this was related to a lower number of CR-ir neurons in the granule cell layer (70 %, p < 0.001) and in the hilus (70 %, p < 0.01) (Supplementary Table 3.1). Contralaterally, at 1 month post-TBI, the number of CR-ir neurons in the molecular layer was 81 % of that at 1 month post-SE (p < 0.01) (Supplementary Table 3.1).
Comparison of TBI and SE: 6-month groups Ipsilaterally, at 6 months post-TBI, the number of CR-ir neurons in the dentate gyrus was 70 % of that at 6 months post-SE (p < 0.001) (Fig. 5a; Supplementary Table 3.1). Laminar analysis revealed that this was related to a lower number of CR-ir neurons in the hilus (61 %, p < 0.001) (Supplementary Table 3.1). Contralaterally, at 6 months post-TBI, the number of CR-ir neurons in the dentate gyrus was 80 % of that at 6 months post-SE (p < 0.05) (Fig. 5a; Supplementary Table 3.1). Laminar analysis revealed that this was related to a lower number of CR-ir neurons in the molecular layer (88 %, p < 0.05) (Supplementary Table 3.1).
CA3 subfield of the hippocampus proper
TBI 1-month group The number of CR-ir neurons in the ipsilateral stratum oriens was 85 % of that in controls (p < 0.05) (Supplementary Table 3.1). Contralaterally, we did not find any differences (Fig. 5b; Supplementary Table 3.1). Also, we did not find any interhemispheric differences (Fig. 5b; Supplementary Table 3.1).
TBI 6-month group Ipsilaterally, the number of CR-ir neurons in the CA3 did not differ from that in controls (Fig. 5b; Supplementary Table 3.1). Contralaterally, the number of CR-ir cells was 81 % of that in controls (p < 0.05) (Fig. 5b; Supplementary Table 3.1), which was related to a reduction in immunopositive neurons within the stratum radiatum (67 % as compared to controls, p < 0.01) (Supplementary Table 3.1). Interhemispheric analysis revealed that the number of CR-ir cells was lower contralaterally in the stratum radiatum (72 % of cells remaining as compared to the ipsilateral side, p < 0.01) (Supplementary Table 3.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of CR-ir neurons did not differ between groups (Fig. 5b; Supplementary Table 3.1). Contralaterally, in the CA3, the number of CR-ir neurons at 6 months was 75 % of that at 1 month post-TBI (p < 0.01) (Fig. 5b; Supplementary Table 3.1). This was related to a reduction in cells of both the stratum oriens (59 %, p < 0.01) and stratum radiatum (66 %, p < 0.001) (Supplementary Table 3.1).
SE 1-month group The number of CR-ir neurons in the CA3 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 3.2). Also, we did not find any interhemispheric differences (Fig. 5b; Supplementary Table 3.2).
SE 6-month group The number of CR-ir neurons assessed in the whole SE group did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 3.2). However, the number of CR-ir neurons in the contralateral pyramidal cell layer was 83 % (p < 0.05) of that ipsilaterally (Supplementary Table 3.2).
SE: comparison of 1-month and 6-month groups The number of CR-ir neurons in the CA3 did not differ between time points either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 3.2).
Comparison of TBI and SE: 1-month groups The number of CR-ir neurons in the CA3 did not differ between groups either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 3.1; Supplementary Table 3.2).
Comparison of TBI and SE: 6-month groups The number of CR-ir neurons in the CA3 did not differ between groups either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 3.1; Supplementary Table 3.2).
CA1 subfield of the hippocampus proper
TBI 1-month group The number of CR-ir neurons in the ipsilateral stratum lacunosum-moleculare was 81 % of that in controls (p < 0.05) (Supplementary Table 3.1). Contralaterally, we did not find any differences (Fig. 5c; Supplementary Table 3.1). Also, we did not find any interhemispheric differences (Fig. 5c; Supplementary Table 3.1).
TBI 6-month group The number of CR-ir neurons in the ipsilateral stratum radiatum was 75 % of that in controls (p < 0.001) (Supplementary Table 3.1). Also, contralaterally, the number of CR-ir neurons was decreased in the stratum radiatum (79 % as compared to controls, p < 0.05) (Supplementary Table 3.1). We did not find any interhemispheric differences (Fig. 5c; Supplementary Table 3.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of CR-ir neurons in the CA1 did not differ between groups (Fig. 5c; Supplementary Table 3.1). Contralaterally, unexpectedly, the 1 month group had a lower number of CR-ir neurons in the stratum lacunosum-moleculare than the 6 months group (72 %, p < 0.05) (Supplementary Table 3.1).
SE 1-month group Ipsilaterally, in controls, the number of CR-ir neurons in the stratum radiatum was 89 % of that at 1 month post-SE (p < 0.05) (Supplementary Table 3.2). Contralaterally, the number of CR-ir neurons in the whole CA1 was 89 % of that in controls (p < 0.05) (Fig. 5c; Supplementary Table 3.2). The number of CR-ir neurons in the contralateral stratum oriens was 81 % of that in controls (p < 0.01) (Supplementary Table 3.2). Also, the number of CR-ir neurons was decreased in the pyramidal cell layer (89 % as compared to controls, p < 0.01). We did not find any interhemispheric differences (Fig. 5c; Supplementary Table 3.2).
SE 6-month group The number of CR-ir neurons assessed in the whole SE group did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5c; Supplementary Table 3.2). However, the number of CR-ir neurons in the contralateral pyramidal cell layer was 88 % (p < 0.05) of that ipsilaterally (Supplementary Table 3.2).
In a subgroup of rats with epilepsy, no differences in any of the cell counts were found when compared to controls or contralateral side (Fig. 5c; Supplementary Table 3.2). However, ipsilaterally, in a subgroup of rats without epilepsy, the number of CR-ir neurons in the stratum radiatum was 87 % of that measured contralaterally (p < 0.05) (Supplementary Table 3.2).
SE: comparison of 1-month and 6-month groups Ipsilaterally, at 1 month post-SE, the number of CR-ir neurons in the stratum oriens was 76 % of that at 6 months post-SE (p < 0.01) (Supplementary Table 3.2). Also at 6 months post-SE, the number of CR-ir neurons in the stratum radiatum was 83 % of that at 1 month post-SE (p < 0.05) (Supplementary Table 3.2). As in the ipsilateral side, contralaterally at 1 month post-SE, the number of CR-ir neurons in the stratum oriens was 74 % of that at 6 months post-SE (p < 0.01) (Supplementary Table 3.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, the number of CR-ir neurons in the CA1 did not differ between groups (Fig. 5c; Supplementary Table 3.1; Supplementary Table 3.2). Contralaterally, at 1 month post-TBI the number of CR-ir neurons in the stratum lacunosum-moleculare was 72 % of that at 1 month post-SE (p < 0.01) (Supplementary Table 3.1).
Comparison of TBI and SE: 6-month groups Ipsilaterally, at 6 months post-TBI, the number of CR-ir neurons in the stratum oriens was 85 % of that at 6 months post-SE (p < 0.05) (Supplementary Table 3.1). Contralaterally, at 6 months post-TBI, the number of CR-ir neurons in the stratum radiatum was 91 % of that at 6 months post-SE (p < 0.05) (Supplementary Table 3.1).
Immunopositive fibers In the TBI group, the immunolabeling of CR-containing fibers in the stratum lacunosum-moleculare of the ipsilateral CA1 was decreased in a patchy manner (data not shown). Also, in the inner molecular layer of the dentate gyrus (Fig. 6), we found a decrease in immunopositive fibers. In the SE model, the decrease in fiber immunolabeling in the stratum lacunosum-moleculare of the CA1 was symmetric (data not shown).
Calretinin (CR) immunoreactivity (ir) in the ipsilateral hippocampus in the different animal groups. Panels on the left are from controls, panels in the middle from rats with TBI 6 months earlier, and panels on the right from rats with SE 6 months earlier (with epilepsy). a A computer-generated plot demonstrating the distribution of CR-ir neurons in the septal hippocampus. Each dot represents one immunopositive neuron. A box with a dashed outline indicates an area from which the photomicrograph in panel C was taken. b CR-ir neurons in the temporal hippocampus. c A representative photomicrograph from the septal end of the dentate gyrus (DG) in a control rat. Arrows show examples of immunopositive neurons. d A computer-generated plot showing the distribution of CR-positive neurons in the septal hippocampus of a rat with TBI 6 months earlier. e Distribution of immunopositive cells in the temporal hippocampus. Note a better preservation of CR-ir neurons in the most temporal hippocampus. f A photomicrograph taken at 6 months post-TBI reveals a decrease in immunopositive neurons in the septal end of the dentate gyrus. Remaining immunopositive neurons (arrows) were mainly in the hilus and in the infragranular region. After SE (a section from a rat with epilepsy), neuronal density was comparable to that in controls both g septally and h temporally. i A photomicrograph of an immunostained section from the septal dentate gyrus. Scale bar equals 100 μm (all photomicrographs)
SOM immunoreactivity
Stereological estimates of SOM-positive neurons in different subfields of the hippocampus proper and dentate gyrus are shown in Online Resource 4 (Supplementary Tables 4.1 and 4.2). Data are shown separately for the ipsilateral and contralateral sides. The pattern of SOM-ir in the ipsilateral hippocampus proper and dentate gyrus in different animal groups is shown in Fig. 7.
Somatostatin (SOM) immunoreactivity (ir) in the ipsilateral hippocampus in the different animal groups. Panels on the left are from controls, panels in the middle from rats with TBI 6 months earlier, and panels on the right from rats with SE 6 months earlier (with epilepsy). a A computer-generated plot demonstrating the distribution of SOM-positive neurons in the septal hippocampus. Each dot represents one immunopositive neuron. A box with a dashed outline indicates an area from which the photomicrograph in panel c was taken. b SOM-ir neurons in the temporal hippocampus. c Representative photomicrograph from the septal dentate gyrus in a control rat. Arrows point to immunopositive neurons. d Computer-generated plot showing the distribution of SOM-positive neurons in the septal hippocampus of a rat with TBI 6 months earlier. Note a remarkable loss on immunopositive neurons in all subfields. e Distribution of immunopositive cells in the temporal hippocampus. Note a better preservation of SOM-positive neurons, particularly in the most temporal hippocampus. f A photomicrograph taken from an immunostained section sampled at 6 months post-TBI shows a decrease in immunopositive neurons in the septal dentate gyrus. After SE (rat with epilepsy), neuronal density was comparable to that in controls both g septally and h temporally. i A photomicrograph of an immunostained section of the septal dentate gyrus. The density of immunopositive neurons (arrows) is comparable to that in controls. Scale bar equals 100 μm (all photomicrographs)
Dentate gyrus
TBI 1-month group The number of SOM-ir neurons in the ipsilateral dentate gyrus was 46 % of that in controls (p < 0.001) (Fig. 5a; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the hilus (43 % of cells remaining as compared to controls, p < 0.001) (Supplementary Table 4.1). Contralaterally, the number of SOM-ir neurons in the hilus was 86 % of that in controls (p < 0.05) (Supplementary Table 4.1). Interhemispheric analysis revealed that the total number of SOM-ir cells in the dentate gyrus was lower ipsilaterally than contralaterally (51 % of cells remaining, p < 0.01) (Fig. 5a; Supplementary Table 4.1). This was related to an ipsilateral reduction in cells both in the granule cell layer (59 % remaining as compared to contralateral side, p < 0.01) and in the hilus (49 %, p < 0.01) (Supplementary Table 4.1).
TBI 6-month group Ipsilaterally, the total number of SOM-ir neurons in the dentate gyrus was 55 % of that in controls (p < 0.001) (Fig. 5a; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells both in the granule cell layer (61 % of cells remaining as compared to controls, p < 0.01) and in the hilus (55 % of cells remaining as compared to controls, p < 0.001) (Supplementary Table 4.1). Contralaterally, we did not find any differences (Fig. 5a; Supplementary Table 4.1). Interhemispheric analysis revealed that the total number of SOM-ir cells in the dentate gyrus was lower ipsilaterally than contralaterally (61 % of cells remaining, p < 0.01) (Fig. 5a; Supplementary Table 4.1). This was related to an ipsilateral reduction in cells of the granule cell layer (66 % remaining as compared to the contralateral side, p < 0.05) and the hilus (60 % remaining as compared to the contralateral side, p < 0.01) (Supplementary Table 4.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of SOM-ir neurons at 6 months post-TBI in the molecular layer was 52 % of that at 1 month post-TBI (p < 0.05) (Supplementary Table 4.1). Contralaterally, the number of SOM-ir neurons at 6 months post-TBI in the molecular layer was 50 % (p < 0.05), and in the granule cell layer was 63 % (p < 0.01) of that at 1 month post-TBI (Supplementary Table 4.1).
SE 1-month group The number of SOM-ir neurons in the dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5a; Supplementary Table 4.2). Interhemispheric analysis revealed that the total number of SOM-ir cells in the granule cell layer was lower ipsilaterally than contralaterally (82 % of cells remaining, p < 0.05) (Supplementary Table 4.2).
SE 6-month group The number of SOM-ir neurons in the dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5a; Supplementary Table 4.2). Also, we did not find any interhemispheric differences (Fig. 5a; Supplementary Table 4.2).
SE: comparison of 1-month and 6-month groups Ipsilaterally, the number of SOM-ir cells at 6 months post-SE in the molecular layer was 32 % (p < 0.01), and in the granule cell layer was 73 % (p < 0.05) of that at 1 month post-SE (Supplementary Table 4.2). Contralaterally, the number of SOM-ir cells at 6 months post-SE in the molecular layer was 35 % (p < 0.01), and in the granule cell layer was 62 % (p < 0.05) of that at 1 month post-SE (Supplementary Table 4.2).
Comparison of TBI and SE: 1-month groups Ipsilaterally, the total number of SOM-ir neurons at 1 month post-TBI in the dentate gyrus was 58 % of that at 1 month post-SE (p < 0.001) (Fig. 5a; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the hilus (55 %, p < 0.001) (Supplementary Table 4.1).
Comparison of TBI and SE: 6-month groups Ipsilaterally, the total number of SOM-ir neurons at 6 month post-TBI in the dentate gyrus was 65 % of that at 6 months post-SE (p < 0.001) (Fig. 5a; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the hilus (64 %, p < 0.001) (Supplementary Table 4.1).
CA3 subfield of the hippocampus proper
TBI 1-month group The number of SOM-ir neurons in the ipsilateral pyramidal cell layer was 80 % (p < 0.05), and in the stratum radiatum was 73 % (p < 0.01) of that in controls (Supplementary Table 4.1). Contralaterally, we did not find any differences (Fig. 5b; Supplementary Table 4.1). We did not find any interhemispheric differences (Fig. 5b; Supplementary Table 4.1).
TBI 6-month group Ipsilaterally, the total number of SOM-ir neurons in the CA3 was 64 % of that in controls (p < 0.01) (Fig. 5b; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in the number of immunopositive cells in both the stratum oriens (57 % of cells remaining as compared to controls, p < 0.001) and the stratum radiatum (67 % of cells remaining as compared to controls, p < 0.05) (Supplementary Table 4.1). Contralaterally, the number of SOM-ir neurons in the stratum oriens was 74 % of that in controls (p < 0.05) (Supplementary Table 4.1). We did not find any interhemispheric differences (Fig. 5b; Supplementary Table 4.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of SOM-ir neurons at 6 months post-TBI in the whole CA3 was 61 % of that at 1 month post-TBI (p < 0.001) (Fig. 5b; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the stratum oriens (57 % of cells remaining as compared to 1 month group, p < 0.01), in the pyramidal cell layer (63 %, p < 0.01) and in the stratum radiatum (64 %, p < 0.01) (Supplementary Table 4.1). Contralaterally, the number of SOM-ir neurons at 6 months post-TBI in the whole CA3 was 58 % (p < 0.001) of that at 1 month post-TBI (Fig. 5b; Supplementary Table 4.1). Laminar analysis revealed that this was related to a loss of immunopositive cells within the stratum oriens (61 % of cells remaining as compared to 1 month group, p < 0.001), in the pyramidal cell layer (48 %, p < 0.001) and in the stratum radiatum (65 %, p < 0.01) (Supplementary Table 4.1).
SE 1-month group The number of SOM-ir neurons in the dentate gyrus did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 4.2). Also, we did not find any interhemispheric differences (Fig. 5b; Supplementary Table 4.2).
SE 6-month group Ipsilaterally, the number of SOM-ir neurons assessed in the whole SE group (including rats with and without epilepsy) in the CA3 did not differ from that in controls (Fig. 5b; Supplementary Table 4.2). Contralaterally, the number of SOM-ir neurons in the whole CA3 was 78 % of that in controls (p < 0.05) (Fig. 5b; Supplementary Table 4.2). Also, contralaterally, the number of SOM-ir cells in the pyramidal cell layer (84 %, p < 0.05) and the stratum radiatum (85 %, p < 0.05) was lower than that ipsilaterally (Supplementary Table 4.2).
Contralaterally, in a subgroup of rats without epilepsy, the number of SOM-ir neurons in the pyramidal cell layer was 82 % (p < 0.05), and in the stratum radiatum 78 % (p < 0.05) of that ipsilaterally (Supplementary Table 4.2).
SE: comparison of 1-month and 6-month groups Ipsilaterally, the total number of SOM-ir neurons at 6 months post-SE in the whole CA3 was 64 % of that at 1 month post-SE (p < 0.001) (Fig. 5b; Supplementary Table 4.2). Laminar analysis revealed that this was related to a reduction in the number of immunopositive cells in the stratum oriens (62 %, p < 0.001), in the pyramidal cell layer (64 %, p < 0.001) and in the stratum radiatum (68 %, p < 0.01) (Supplementary Table 4.2). Contralaterally, the total number of SOM-ir neurons at 6 months post-SE in the whole CA3 was 59 % of that at 1 month post-SE (p < 0.001) (Fig. 5b; Supplementary Table 4.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the stratum oriens (60 %, p < 0.01), the pyramidal cell layer (61 %, p < 0.001) and of the stratum radiatum (55 %, p < 0.001) (Supplementary Table 4.2).
Comparison of TBI and SE: 1-month groups Contralaterally, the total number of SOM-ir neurons at 1 month post-SE in the whole CA3 was 86 % of that at 1 month post-TBI (p < 0.01) (Fig. 5b; Supplementary Table 4.2).
Comparison of TBI and SE: 6-month groups The number of SOM-ir neurons in the CA3 did not differ between groups either ipsilaterally or contralaterally (Fig. 5b; Supplementary Table 4.1; Supplementary Table 4.2).
CA1 subfield of the hippocampus proper
TBI 1-month group The number of SOM-ir neurons in the CA1 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5c; Supplementary Table 4.1). Also, we did not find any interhemispheric differences (Fig. 5c; Supplementary Table 4.1).
TBI 6-month group Ipsilaterally, the total number of SOM-ir neurons in the CA1 was 75 % of that in controls (p < 0.01) (Fig. 5c; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the stratum oriens (74 % of cells remaining as compared to controls, p < 0.01) (Supplementary Table 4.1). Contralaterally, the total number of SOM-ir neurons in the CA1 was 78 % of that in controls (p < 0.001) (Fig. 5c; Supplementary Table 4.1). Laminar analysis revealed that this was connected with a reduction in immunopositive cells in both the stratum oriens (81 % of cells remaining as compared to controls, p < 0.01) and the pyramidal cell layer (64 %, p < 0.01) (Supplementary Table 4.1). We did not find any interhemispheric differences (Fig. 5c; Supplementary Table 4.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of SOM-ir neurons at 6 months post-TBI in the stratum radiatum was 54 % of that at 1 month post-TBI (p < 0.01) (Supplementary Table 4.1). Contralaterally, the number of SOM-ir neurons at 6 months post-TBI in the whole CA1 was 74 % (p < 0.01) of that at 1 month post-TBI (Fig. 5c; Supplementary Table 4.1). Laminar analysis revealed that this was related to a reduction in the number of immunopositive cells in the stratum oriens (80 % of cells remaining as compared to the 1-month group, p < 0.05), in the pyramidal cell layer (53 %, p < 0.001) and in the stratum radiatum (58 %, p < 0.05) (Supplementary Table 4.1).
SE 1-month group Ipsilaterally, we did not find any differences (Fig. 5c; Supplementary Table 4.2). Contralaterally, the total number of SOM-ir neurons in the CA1 was 86 % of that in controls (p < 0.05) (Fig. 5c; Supplementary Table 4.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells of both the stratum oriens (84 % of cells remaining as compared to controls, p < 0.05) and stratum radiatum (76 %, p < 0.05) (Supplementary Table 4.2). We did not find any interhemispheric differences (Fig. 5c; Supplementary Table 4.2).
SE 6-month group The number of SOM-ir neurons in the CA1 assessed in the whole SE group (including rats with and without epilepsy) did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5c; Supplementary Table 4.2). Contralaterally, the number of SOM-ir neurons in the whole CA1 was 91 % of that in ipsilaterally (p < 0.05) (Fig. 5c; Supplementary Table 4.2). Also, contralaterally, the number of SOM-ir cells in the pyramidal cell layer was 72 % of that ipsilaterally (p < 0.05) (Supplementary Table 4.2).
SE: comparison of 1-month and 6-month groups Ipsilaterally, the total number of SOM-ir neurons at 6 months post-SE in the whole CA1 was 79 % of that at 1 month post-SE (p < 0.01) (Fig. 5c; Supplementary Table 4.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the pyramidal cell layer (51 %, p < 0.001) and within the stratum radiatum (39 %, p < 0.001) (Supplementary Table 4.2). Contralaterally, the total number of SOM-ir neurons at 6 months post-SE in the whole CA1 was 75 % of that at 1 month post-SE (p < 0.001) (Fig. 5c; Supplementary Table 4.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the pyramidal cell layer (37 %, p < 0.001) and of the stratum radiatum (34 %, p < 0.001) (Supplementary Table 4.2).
Comparison of TBI and SE: 1-month groups Contralaterally the total number of SOM-ir neurons at 1 month post-SE in the whole CA3 was 77 % of that at 1 month post-TBI (p < 0.01) (Fig. 5c; Supplementary Table 4.2). Laminar analysis revealed that this was related to a reduction in the number of immunopositive cells in the stratum oriens (74 % of cells remaining as compared to the 1 month post-TBI group, p < 0.01) (Supplementary Table 4.2).
Comparison of TBI and SE: 6-month groups Ipsilaterally, the number of SOM-ir neurons at 6 months post-SE in the pyramidal cell layer was 66 % of that at 6 months post-TBI (p < 0.01) (Supplementary Table 4.2). Contralaterally, the number of SOM-ir neurons at 6 months post-SE in the whole CA1 was 78 % (p < 0.001) of that at 6 months post-TBI (Fig. 5c; Supplementary Table 4.2). Laminar analysis revealed that this was related to a loss of immunopositive cells from within the stratum oriens (82 %, p < 0.01) and the pyramidal cell layer (58 %, p < 0.01) (Supplementary Table 4.2).
NPY immunoreactivity
Stereological estimates of NPY-positive neurons in different subfields of the hippocampus proper and dentate gyrus are shown in Online Resource 5 (Supplementary Tables 5.1 and 5.2). Data are shown separately for the ipsilateral and contralateral sides.
No stereological counting of cells was done at 1 month post-SE as the intensive immunoreactivity in the mossy fiber pathway in 29 % (4 of 14) of animals with SE biased the cell counting in the CA3 and the dentate gyrus (Fig. 8m, n). In the CA1, there was intensive NPY-positive immunoreactivity in the pyramidal cell layer (Fig. 8o).
Neuropeptide Y (NPY) immunoreactivity (ir) in the ipsilateral hippocampus in the different animal groups. Panels of the left are computer-generated plots from controls, panels in the middle from rats with TBI 6 months earlier, and panels on the right from rats with SE (with epilepsy) 6 months earlier. Columns of photomicrographs show immunoreactivity (from left to right) in controls, 6 months post-TBI, 1 month post-SE, and 6 months post-SE (rats with epilepsy). a A computer-generated plot demonstrating the distribution of NPY-positive neurons in the septal hippocampus. Each dot represents one immunopositive neuron. A box with a dashed outline indicates an area from which the photomicrograph in panel c was taken. b NPY-positive neurons in the temporal hippocampus. Boxes with dashed outlines indicate regions from which the photomicrographs in panel d (b1) and e (b2) were taken. c A representative photomicrograph of the septal dentate gyrus in a control rat. The density of NPY-ir neurons (black arrows) was high. Note the lack of immunostaining in the granule cell layer (white arrow) and the hilus (open arrow). d Distribution of NPY immunoreactivity in the temporal end of the dentate gyrus. e NPY immunoreactivity in the CA1. f A computer-generated plot showing the distribution of NPY-positive neurons in the septal hippocampus of a rat with TBI 6 months earlier. Note a loss on immunopositive neurons in the dentate gyrus and CA3 (particularly in the stratum radiatum). g Distribution of immunopositive cells in the temporal hippocampus. Note a slightly better preservation of NPY-positive neurons in the most temporal region of the hippocampus and in the dentate gyrus as compared to the septal end. h A photomicrograph taken from an immunostained section of the septal dentate gyrus sampled at 6 months post-TBI. Very few immunopositive cells (black arrow) were remaining. A remarkable loss of immunopositive neurons was also found in the i temporal end of the dentate gyrus and j in the CA1. Like in controls, no immunostaining was found in the granule cell layer (white arrow) or the hilus (open arrow). At 6 months post-SE (a rat with epilepsy), neuronal density was decreased as compared to controls both k septally and l temporally. m A representative photomicrograph from an immunostained section of the septal dentate gyrus at 1 month post-SE. Unlike in controls or rats with TBI, there was an intense NPY-ir labeling in the granule cell layer (white arrow) and the hilus (open arrow) after SE. n Also in the temporal dentate gyrus, the density of NPY-ir was high in the granule cell layer (white arrow) and in the hilus (open arrow). o Increased NPY-immunoreactivity surrounded the pyramidal cells in the CA1 (thick arrow) at 1 month post-SE (compare panels o and e). p A photomicrograph from an immunostained section of the septal dentate gyrus from a rat with epilepsy, showing a loss of NPY immunoreactive neurons (black arrow) as compared to controls. Unlike at 1 month post-SE, only the hilus but not the granule cell layer remained intensely immunostained (compare panels p and m). q Also in the temporal dentate gyrus, the hilus was intensely stained (open arrow). r In the CA1, staining was comparable to that in controls (black arrow and thick arrow). e Scale bar equals 100 μm (all photomicrographs). GCL granule cell layer, H hilus
Dentate gyrus
TBI 1-month group Ipsilaterally, the total number of NPY-ir neurons in the whole dentate gyrus was 59 % of that in controls (p < 0.001) (Fig. 5a; Supplementary Table 5.1). Laminar analysis revealed that this was related to a reduction in immunopositive cell numbers in the molecular layer (74 % of cells remaining as compared to controls, p < 0.01) and in the hilus (52 %, p < 0.001) (Supplementary Table 5.1). Contralaterally, in controls, the number of NPY-ir cells in the dentate gyrus was 91 % of that in the TBI group (p < 0.05) (Fig. 5a; Supplementary Table 5.1).
Ipsilaterally, at 1 month post-TBI, the number of NPY-ir cells in the whole dentate gyrus was 56 % of that contralaterally (p < 0.01) (Fig. 5a; Supplementary Table 5.1), which was related to a reduction in immunopositive neurons in the molecular layer (63 %, p < 0.01), the granule cell layer (84 %, p < 0.01) and in the hilus (51 %, p < 0.01) (Supplementary Table 5.1).
TBI 6-month group Ipsilaterally, the total number of NPY-ir neurons in the whole dentate gyrus was 51 % of that in controls (p < 0.001) (Fig. 5a; Supplementary Table 5.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the granule cell layer (66 % of cells remaining as compared to controls, p < 0.01) and within the hilus (46 %, p < 0.001) (Supplementary Table 5.1). Contralaterally, we did not find any differences (Fig. 5a; Supplementary Table 5.1).
Ipsilaterally, at 6 months post-TBI, the number of NPY-ir cells in the whole dentate gyrus was 58 % of that contralaterally (p < 0.05) (Fig. 5a; Supplementary Table 5.1), which was related to a reduction in the number of immunopositive neurons in the granule cell layer (64 %, p < 0.05) and in the hilus (55 %, p < 0.05) (Supplementary Table 5.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of NPY-ir neurons in the dentate gyrus at 6 months post-TBI was 83 % of that at 1 month post-TBI (p < 0.05) (Fig. 5a; Supplementary Table 5.1), which was related to a reduction of immunopositive neurons in the granule cell layer (78 %, p < 0.05) (Supplementary Table 5.1). Contralaterally, the number of NPY-ir neurons at 6 months post-TBI was 81 % of that at 1 month post-TBI (p < 0.05) (Fig. 5a; Supplementary Table 5.1). The decrease present in the molecular layer was 50 % (p < 0.001) and in the hilus, 80 % (p < 0.01) (Supplementary Table 5.1).
SE 6-month group Ipsilaterally, the number of NPY-ir neurons assessed in the whole SE group (including rats with and without epilepsy) in the granule cell layer was 62 % of that in controls (p < 0.05) (Supplementary Table 5.2). The number of NPY-ir neurons in the ipsilateral granule cell layer was 90 % of that contralaterally (p < 0.05) (Supplementary Table 5.2).
Ipsilaterally, in a subgroup of rats with epilepsy, the total number of NPY-ir neurons in the whole dentate gyrus was 33 % of that in controls (p < 0.01) (Fig. 5a; Supplementary Table 5.2). Laminar analysis revealed that this was related to a reduction in immunopositive cell counts in the molecular layer (57 % of cells remaining as compared to controls, p < 0.05), in the granule cell layer (35 %, p < 0.01) and in the hilus (31 %, p < 0.01) (Supplementary Table 5.2). Contralaterally, the number of NPY-ir cells in the dentate gyrus was 37 % of that in controls (p < 0.01) (Fig. 5a; Supplementary Table 5.2), which was related to a reduction in immunopositive neurons within the granule cell layer (42 %, p < 0.01) and the hilus (35 %, p < 0.01) (Supplementary Table 5.2). Ipsilaterally, in a subgroup of rats with epilepsy, the number of NPY-ir cells in the granule cell layer was 83 % of that contralaterally (p < 0.05) (Supplementary Table 5.2).
When rats with and without seizures were compared, we found that in a subgroup of rats with epilepsy, the total number of NPY-ir neurons in the whole dentate gyrus was 36 % of that in rats without epilepsy at 6 months post-SE (p < 0.05) (Fig. 5a; Supplementary Table 5.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the granule cell layer (41 % of cells remaining as compared to rats without epilepsy at 6 months post-SE, p < 0.05) and in the hilus (33 %, p < 0.05) (Supplementary Table 5.2). Contralaterally, also in a subgroup of rats with epilepsy, the number of NPY-ir cells in the dentate gyrus was 41 % of that in rats without epilepsy at 6 months post-SE (p < 0.05) (Fig. 5a; Supplementary Table 5.2), which was related to a reduction in the number of immunopositive neurons in the granule cell layer (45 %, p < 0.01) (Supplementary Table 5.2).
Comparison of TBI and SE: 6-month groups Contralaterally, the number of NPY-ir neurons in the granule cell layer in the SE group was 64 % of that in the TBI group (p < 0.05) (Supplementary Table 5.2).
Immunopositive axons At 1 month post-SE, 29 % of animals had intense immunoreactivity in the mossy fiber pathway bilaterally (data not shown). At 6 months, all except one of the animals with spontaneous seizures after SE had an immunopositive mossy fiber pathway bilaterally (data not shown). Only one of those without spontaneous seizures after SE had an immunopositive mossy fiber pathway (data not shown). In line with these observations, we found increased immunopositivity in the tip and crest portions of the inner molecular layer at 6 months post-SE in animals with spontaneous seizures, suggesting NPY-immunoreactivity in sprouted mossy fibers (data not shown). Importantly, none of the animals with TBI assessed at either 1 or 6 months post-injury had an immunopositive mossy fiber pathway (data not shown). We also noticed that despite about a 50 % reduction in the number of hilar immunopositive cells, there was intense axonal labeling in the outer two-thirds of the molecular layer, suggesting sprouting of the axons of the remaining NPY-positive cells (data not shown).
CA3 subfield of the hippocampus proper
TBI 1-month group The number of NPY-ir neurons in the ipsilateral pyramidal cell layer was 88 % of that in controls (p < 0.05) (Supplementary Table 5.1). Contralaterally, we did not find any differences (Fig. 5b; Supplementary Table 5.1). Also, we did not find any interhemispheric differences (Fig. 5b; Supplementary Table 5.1).
TBI 6-month group Ipsilaterally, the total number of NPY-ir neurons in the whole CA3 was 76 % of that in controls (p < 0.01) (Fig. 5b; Supplementary Table 5.1). Laminar analysis revealed that this was related to a reduction in immunopositive cells of the pyramidal cell layer (77 % of cells remaining as compared to controls, p < 0.05) and of the stratum radiatum (73 %, p < 0.01) (Supplementary Table 5.1). Contralaterally, we did not find any differences (Fig. 5b; Supplementary Table 5.1). Also, we did not find any interhemispheric differences (Fig. 5b; Supplementary Table 5.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of NPY-ir neurons in the CA3 at 6 months post-TBI was 73 % of that at 1 month post-TBI (p < 0.001) (Fig. 5b; Supplementary Table 5.1), which was related to a reduction in immunopositive neurons within the stratum oriens (73 %, p < 0.01), the pyramidal cell layer (77 %, p < 0.05) and the stratum radiatum (68 %, p < 0.001) (Supplementary Table 5.1). Contralaterally, the number of NPY-ir neurons at 6 months in the whole CA3 was 76 % of that at 1 month post-TBI (p < 0.01) (Fig. 5b; Supplementary Table 5.1), which was related to a reduction in immunopositive neurons of the stratum oriens (76 %, p < 0.05), the pyramidal cell layer (76 %, p < 0.05) and the stratum radiatum (76 %, p < 0.05) (Supplementary Table 5.1).
SE 6-month group Ipsilaterally, the number of NPY-ir neurons in the whole CA3 assessed in the SE group (including rats with and without epilepsy) was 75 % of that in controls (p < 0.05) (Fig. 5b; Supplementary Table 5.2). Contralaterally, we did not find any differences (Fig. 5b; Supplementary Table 5.2).
Ipsilaterally, in a subgroup of rats with epilepsy, the total number of NPY-ir neurons in the whole CA3 was 54 % of that in controls (p < 0.01) (Fig. 5b; Supplementary Table 5.2). Laminar analysis revealed that this was related to a reduction in immunopositive cells within the stratum oriens (60 % of cells remaining as compared to controls, p < 0.05), the pyramidal cell layer (41 %, p < 0.01) and the stratum radiatum (65 %, p < 0.05) (Supplementary Table 5.2). Contralaterally, the number of NPY-ir cells in the whole CA3 was 57 % of that in controls (p < 0.05) (Fig. 5b; Supplementary Table 5.2), which was related to a reduction in immunopositive neurons within the pyramidal cell layer (44 %, p < 0.01) (Supplementary Table 5.2). Contralaterally, the number of NPY-ir neurons in the pyramidal cell layer was 90 % of that seen ipsilaterally (p < 0.05) (Supplementary Table 5.2).
Comparison of TBI and SE: 6-month groups Contralaterally, the number of NPY-ir neurons at 6 months post-SE was 72 % of that at 6 months post-TBI (p < 0.05) (Fig. 5b; Supplementary Table 5.2), which was related to a reduction in immunopositive neurons of the stratum oriens (68 %, p < 0.05) (Supplementary Table 5.2).
CA1 subfield of the hippocampus proper
TBI 1-month group In the control group, the number of NPY-ir neurons in the ipsilateral stratum oriens was 87 % of that in the TBI group (p < 0.05) (Supplementary Table 5.1). Contralaterally, we did not find any differences (Fig. 5c; Supplementary Table 5.1). Also, we did not find any interhemispheric differences (Fig. 5c; Supplementary Table 5.1).
TBI 6-month group The number of NPY-ir neurons in the CA1 did not differ from that in controls either ipsilaterally or contralaterally (Fig. 5c; Supplementary Table 5.1). However, the number of NPY-ir neurons in the ipsilateral CA1 was 92 % of that contralaterally (p < 0.05) (Fig. 5c; Supplementary Table 5.1), which was related to fewer cells in the pyramidal cell layer (88 %, p < 0.05) (Supplementary Table 5.1).
TBI: comparison of 1-month and 6-month groups Ipsilaterally, the number of NPY-ir neurons in the CA3 at 6 months post-TBI was 76 % of that at 1 month post-TBI (p < 0.01) (Fig. 5c; Supplementary Table 5.1), which was related to a reduction in immunopositive neurons within the stratum oriens (75 %, p < 0.05), within the pyramidal cell layer (82 %, p < 0.01) and within the stratum radiatum (76 %, p < 0.05) (Supplementary Table 5.1). Contralaterally, the number of NPY-ir neurons at 6 months was 87 % of that at 1 month post-TBI (p < 0.01) (Fig. 5c; Supplementary Table 5.1), which was related to a reduction in immunopositive neurons of the stratum oriens (88 %, p < 0.05), the pyramidal cell layer (92 %, p < 0.01) and the stratum radiatum (84 %, p < 0.05) (Supplementary Table 5.1).
SE 6-month group Ipsilaterally, the number of NPY-ir neurons assessed in the whole SE group (including rats with and without epilepsy) in the stratum radiatum was 81 % of that in controls (p < 0.05) (Supplementary Table 5.2). Contralaterally, we did not find any differences (Fig. 5c; Supplementary Table 5.2).
Ipsilaterally, in a subgroup of rats with epilepsy, the number of NPY-ir neurons in the stratum radiatum was 77 % of that in controls (p < 0.05) (Supplementary Table 5.2).
Comparison of TBI and SE: 6-month groups The number of NPY-ir neurons in the CA1 did not differ between groups either ipsilaterally or contralaterally (Fig. 5c; Supplementary table 5.1; Supplementary Table 5.2).
Interneuron loss and seizure susceptibility after TBI
Only those neuronal counts that showed a statistically significant change as compared to corresponding controls were included in the correlation analysis. Analysis was done hierarchically by first calculating the correlations between the indices of seizure excitability and the total number of neurons bilaterally, and then, progressing to correlations with ipsilateral/contralateral cell counts, to correlations with perisomatic or dendritic neurons, and eventually to correlations with counts of different neuronal populations in individual hippocampal subfields (Online Resource 6).
All interneurons
We found only one parameter in the PTZ test to correlate with the total number of all 5 investigated interneuron subclasses at 6 months post-TBI, and even that was towards an unexpected direction, as the lower the number of neurons in the contralateral dentate gyrus, the lower was the number of epileptiform spikes (r = 0.857, n = 7, p < 0.05) (Supplementary Table 6.1).
Perisomatic interneurons
Total number of all perisomatic interneurons (i.e., sum of the total number of PARV and CCK-positive cells) ipsilaterally and/or contralaterally did not correlate with seizure susceptibility (Supplementary Table 6.2).
Next, we assessed if a reduction in perisomatic interneurons in different subfields would indicate any associations between the cell loss and hyperexcitability. We found that the lower the number of PARV-ir neurons in the ipsilateral stratum radiatum of the CA3, the higher was the number of EDs in the PTZ test (r = −0.719, p < 0.05) (Supplementary Table 6.3). However, the lower the number of PARV-ir neurons in the contralateral granule cell layer, the lower was the number of epileptiform spikes in the PTZ test at 6 months post-TBI (r = 0.695, p < 0.05) (Supplementary Table 6.3).
All correlations calculated for CCK-ir neurons were unexpected. The lower the number of CCK-ir neurons in the contralateral dentate gyrus or in the contralateral hilus only, the lower was the number of epileptiform spikes in the PTZ test (r = 0.826, p < 0.05 and r = 0.850, p < 0.01, respectively) (Supplementary Table 6.3). Also, the lower the number of CCK-ir neurons in these regions, the longer was the latency to the first ED in the PTZ test (contralateral dentate gyrus r = −0.826, p < 0.05; hilus r = −0.898, p < 0.01) (Supplementary Table 6.3). The lower the number of CCK-ir neurons ipsilaterally in the stratum oriens, the longer was the latency to the first ED in the PTZ test (r = −0.759, n = 8, p < 0.05) (Supplementary Table 6.3). Finally, the lower the number of CCK-ir neurons ipsilaterally in the stratum oriens or stratum radiatum, the lower was the number of epileptiform spikes in the PTZ test (r = 0.952, n = 8, p < 0.001; r = 0.719, n = 8, p < 0.05; respectively) (Supplementary Table 6.3).
Dendritic interneurons
The total number of all dendritic interneurons (i.e., the sum of the CR, SOM and NPY-positive cells ipsilaterally and/or contralaterally) did not correlate with any of the indices of seizure susceptibility (Supplementary Table 6.2).
When individual neuronal classes were analyzed, we found that the lower the total number of CR-ir neurons in the contralateral hippocampus, the longer was the mean duration of the EDs (r = −0.745, n = 9, p < 0.05) (Supplementary Table 6.4). Analysis of different hippocampal subfields indicated that the lower the number of CR-ir neurons ipsilaterally in the hilus, the longer was the mean duration of the ED (r = −0.745, n = 9, p < 0.05) (Supplementary Table 6.4). The lower the number of CR-ir neurons contralaterally in the stratum radiatum of the CA1, the higher was the number of EDs (r = −0.828, n = 9, p < 0.01) (Supplementary Table 6.4).
Analysis of SOM-ir neurons indicated that the lower the total number of SOM-ir neurons in both hemispheres, the longer was the latency to the first epileptiform spike (r = −0.678, n = 9, p < 0.05) (Supplementary Table 6.4). Also, the lower the total number of SOM-ir neurons in the ipsilateral hippocampus, the longer was the latency to the first epileptiform spike (r = −0.795, n = 9, p < 0.05) and the first ED (r = −0.700, n = 9, p < 0.05) (Supplementary Table 6.4). The lower the number of SOM-ir neurons ipsilaterally in the dentate gyrus or in the hilus only, the higher was the number of EDs (r = 0.717, n = 9, p < 0.05; r = 0.733, n = 9, p < 0.05, respectively) (Supplementary Table 6.4). However, the lower the number of SOM-ir neurons ipsilaterally in the whole CA3 or in the stratum oriens only, the longer was the latency to first epileptiform spike in the PTZ test (r = −0.720, n = 9, p < 0.05; r = −0.711, n = 9, p < 0.05, respectively) (Supplementary Table 6.4). The lower the number of SOM-ir neurons contralaterally in the pyramidal cell layer of the CA1, the shorter was the latency to first ED (r = 0.700, n = 9, p < 0.05) (Supplementary Table 6.4). The lower the number of SOM-ir neurons contralaterally in the pyramidal cell layer, the higher was the number of epileptiform spikes (r = −0.683, n = 9, p < 0.05) (Supplementary Table 6.4). However, the lower the number of SOM-ir neurons contralaterally in CA1 or in the stratum oriens only, the shorter was the mean duration of the ED (r = 0.686, n = 9, p < 0.05; r = 0.745, n = 9, p < 0.05, respectively) (Supplementary Table 6.4).
Analysis of NPY-ir neurons revealed that the lower the number of NPY-ir neurons in the ipsilateral dentate gyrus, the longer was the latency to the first spike (−0.810, n = 7, p < 0.05) (Supplementary Table 6.4). The lower the number of NPY-ir neurons ipsilaterally in the granule cell layer, the longer the latency to the first ED (r = −0.778, n = 8, p < 0.05) and the lower was the number of epileptiform spikes (r = 0.755, n = 8, p < 0.05) (Supplementary Table 6.4). The lower the number of NPY-ir neurons in the pyramidal cell layer of the CA3, the longer was the latency to the first spike (−0.857, n = 7, p < 0.01) and ED (−0.833, n = 7, p < 0.05) (Supplementary Table 6.4).
Interneuron loss and seizure susceptibility after SE
We found that the lower the total number of all interneuron subclasses (both ipsilaterally and contralaterally), the higher was the number of seizures in animals with post-SE epilepsy (r = −0.829, n = 6, p < 0.05) (Supplementary Table 7.1). Also, the lower the sum of all interneuron subclasses in the ipsilateral hippocampal proper (CA3 and CA1), the higher was the number of seizures (r = −0.829, n = 6, p < 0.05) (Supplementary Table 7.1).
Perisomatic interneurons
A reduction in the total number of PARV-ir or CCK-ir neurons did not correlate with the mean duration of spontaneous seizures or seizure frequency at 6 months post-SE (Supplementary Table 7.2).
Dendritic interneurons
The lower the total number of all dendritic interneurons (sum of CR-ir, SOM-ir and NPY-ir neurons both ipsilaterally and contralaterally), the higher was the number of spontaneous seizures at 6 months post-SE (r = −0.829, n = 6, p < 0.05) (Supplementary Table 7.2). When individual subclasses of dendritic neurons were analyzed, we did not find any associations between reductions in neuronal counts and the duration or frequency of spontaneous seizures.
The only subfield-specific correlation revealed that the lower the number of NPY-ir neurons in the ipsilateral molecular layer of the dentate gyrus, the higher was the number of spontaneous seizures (r = −0.889, n = 6, p < 0.05) (Supplementary Table 7.3).
Other histological analyses
To further address a question of whether the remarkable loss of immunostained interneurons after TBI related to their death rather than a change in their chemotype, we assessed the neurodegeneration in the hilus in more detail. Firstly, unbiased stereological estimation demonstrated that only 53 % of the hilar cells was remaining ipsilaterally at 1 month post-TBI (p < 0.05 as compared to controls) (Fig. 9e). Secondly, WFA-positive perineuronal nets (indicating PARV-ir neurons) around Fluoro-Jade B positive cells indicated the death of PARV-ir neurons both in the hilus and in the CA1 stratum oriens at 4 h post-TBI (Fig. 9f–i). Thirdly, qualitative analysis of SPR immunohistochemistry revealed a loss of immunopositive neurons which was more pronounced when the reduction in PARV-ir neuronal numbers was greater (Online resource 8).
Hippocampal neurodegeneration after lateral fluid-percussion injury (FPI) at 1 month post-TBI. Coronal thionin-stained sections from the septal hippocampus a in a control rat and b in a rat with lateral FPI. Principal cell layers were relatively well-preserved. Typically, only a mild loss of principal cells in the CA3a was detected septally (arrow). Higher magnification photomicrographs from the hilus in c a control animal and d in a rat with TBI. Note a remarkable loss of hilar neurons and gliosis in the hilus of an injured rat (arrow in d). e A graph summarizing the data from a stereological assessment of the total number of hilar neurons in thionin-stained preparations (black bars) and the total number immunopositive interneurons in various immunopreparations (sum of PARV, CCK, CR, SOM, and NPY-positive cells, white bars) ipsilaterally and contralaterally in controls and injured rats. Ipsilaterally, the total number of hilar neurons was 51 % of that in controls (p < 0.01). The total number of interneurons was 55 % of that in controls (p < 0.01). Contralaterally, neuronal numbers were comparable to those of controls. f Labeling of a WFA-positive perineuronal net (indicating a PARV-positive interneuron; Härtig et al. 1992) in the hilus at 4 h post-TBI. g Double-labeling of the WFA-positive perineuronal net with Fluoro-Jade B (arrow), indicating degeneration of a PARV-positive cell. h WFA-positive perineuronal net in the stratum oriens of the CA1 at 4 h post-TBI. i Double-labeling of the WFA-positive perineuronal net in the CA1 with Fluoro-Jade B (arrow), indicating degeneration of a PARV-positive cell. DG dentate gyrus, GCL granule cell layer, H hilus, MOL molecular layer. Statistical significances: **p < 0.01 (Mann–Whitney U test). Scale bars equal 400 μm in panels a and b, 100 μm in c and d, and 10 μm in f–i
Discussion
Even though a reduction in the number of hippocampal interneurons is present in experimental models of acquired epilepsy and in human TLE (Mathern et al. 1995; Wasterlain et al. 1996; Maglóczky and Freund 2005; Tóth et al. 2010), a link between the severity of interneuron loss and epilepsy phenotype is still, however, poorly explored. The present study tested a hypothesis that the occurrence and severity of epilepsy depend on the magnitude of the reduction in hippocampal GABAergic interneurons. We compared two models of acquired focal epilepsy triggered by SE or TBI. The numbers of five different subpopulations of hippocampal GABAergic neurons were assessed at 1 or 6 months post-insult. The severity of epilepsy was assessed with video-EEG and with the PTZ seizure susceptibility test. The key finding was that in rats with TBI with no spontaneous seizures during 2 weeks of video-EEG monitoring at 6 months post-injury, the loss of GABAergic interneurons was remarkably greater and more widespread than in rats that developed epilepsy after SE.
Methodological considerations
To assess whether rats had developed epilepsy, we used a 2-week continuous video-EEG recording to detect spontaneous seizures. Related to the limited monitoring period, it is possible that some animals with a very low seizure frequency were misdiagnosed to be “non-epileptic”. However, this would not change the overall conclusion of the study indicating a lack of correlation between the number of hippocampal interneurons and seizure susceptibility.
It is still under discussion if a PTZ seizure susceptibility test can be used to diagnose ongoing epileptogenesis. However, PTZ test is commonly used to assess seizure susceptibility and seizure threshold in normal and genetically modified rodents (Löscher and Nolting 1991). Also, studies both in our laboratory (Kharatishvili et al. 2007; Bolkvadze and Pitkänen 2012) and elsewhere (Blanco et al. 2009; Rattka et al. 2011) have shown a link between the hyperexcitability in a PTZ test and development of spontaneous seizures.
Stereological analysis indicated that the numbers of PARV-ir, CCK-ir, CR-ir, SOM-ir, and NPY-ir neurons were remarkably decreased already at 1 month post-TBI, being greatest in the ipsilateral dentate gyrus where only 46–77 % of immunopositive neurons remained. As the reduction in the immunopositive cell counts could result from a reduced expression of the epitope rather than the death of interneurons (Sloviter 1991; Wittner et al. 2001; Sun et al. 2007), we performed three control experiments in the TBI model in which the loss of immunoreactivity was more robust than after SE. Firstly, we estimated the total number of hilar neurons at 1 month post-TBI to obtain a general idea of the severity of neurodegeneration. Similar to previous studies in adult Sprague–Dawley rats (Buckmaster and Dudek 1997; Buckmaster and Jongen-Rêlo 1999; Thind et al. 2010), the mean number of hilar neurons in controls was around 47,000. At 1 month post-TBI, about 24,900 (53 %) neurons were remaining ipsilaterally (i.e., about 22,100 neurons had died by 1 month) whereas contralaterally the neuronal number did not differ from that in controls, which is in line with previous observations in the lateral FPI model (Lowenstein et al. 1992; Toth et al. 1997). Calculation of the sum of hilar PARV, CCK, SOM, CR, and NPY-positive neurons revealed a total of 26,600 interneurons in controls and 14,700 in rats with TBI, suggesting that about 12,000 interneurons had died by 1 month post-TBI, which could explain about a half of the hilar cell loss. It should be noted that as we counted the sum of all interneurons, and did not take into account the partial overlap of different neuronal markers (for example see Bering et al. 1997; Dinocourt et al. 2003; Sloviter et al. 2003), it is possible that the total neuronal number was overestimated. Also, mossy cells that form about 40 % of the total neuronal population in the hilus are also damaged by lateral FPI, which could explain at least part of the reduction in hilar neuronal numbers (Toth et al. 1997). Therefore, we needed more direct evidence for the interneuronal death observed.
We next assessed whether the most remarkably affected GABAergic neuronal population, PARV-ir neurons, co-label with a neurodegeneration marker at the early post-TBI phase. As PARV-ir neurons contain WFA-positive perineuronal nets (Härtig et al. 1992), it became possible to perform double-labeling with intraneuronally located Fluoro-Jade B and perineuronally located WFA-ir to demonstrate degeneration of PARV-ir neurons (Karetko-Sysa et al. 2011). We found that in samples collected at 4 h post-TBI, a subpopulation of Fluoro-Jade B positive neurons were surrounded by WFA-positive perineuronal nets both in the dentate gyrus and the hippocampus proper. Moreover, we counted the percentage of 50 PARV-ir cells which were also Fluoro-Jade B positive in the adjacent lesioned cortex at 4 h post-TBI. Analysis of 2 rats showed that in Rat #1 42 % (21/50) and in Rat #2 34 % (17/50) of PARV-ir neurons were Fluoro-Jade B positive, indicating their degeneration. As Fluoro-Jade B labels irreversibly damaged neurons (Schmued and Hopkins 2000), our data indicate that the loss of interneurons containing PARV is due to cell death rather than a reduction in the epitope expression.
Finally, sections adjacent to those used for PARV immunohistochemistry from controls and animals with TBI at 1 or 6 months earlier were stained with an antibody raised against SPR, a marker of hippocampal PARV-ir neurons (Sloviter et al. 2001, 2003). We found that more pronounced the reduction in PARV-ir neurons, more pronounced the reduction in SPR-ir neurons, representing another type of proof for death of PARV-ir neurons after TBI.
Loss of interneurons responsible of perisomatic inhibition after SE and TBI
Parvalbumin-immunoreactive interneurons
Lateral FPI-induced TBI results in an increased seizure susceptibility in about 80 % of rats and leads to epilepsy in about 50 % of rats as shown in a year-long follow-up study (Kharatishvili et al. 2006). As our previous intra-hippocampal EEG recordings have demonstrated, the hippocampus is activated during spontaneous seizures (Kharatishvili et al. 2006). In the present study, rats with lateral FPI developed increased seizure susceptibility but did not express spontaneous seizures during a 2-week video-EEG monitoring period. Yet, there was a remarkable reduction in PARV-ir neurons in different hippocampal subfields that was visible already at 1 month post-TBI as only 62–82 % of PARV-ir neurons were remaining in the ipsilateral dentate gyrus or CA1. By 6 months post-TBI, the degeneration of PARV-ir neurons had progressed, being present in different hippocampal subfields bilaterally with only 35–67 % of neurons remaining as compared to that in controls. Moreover, by 6 months, the loss of immunoreactivity extended throughout the septo-temporal axis of the hippocampus, even though it was still more pronounced septally. There are three previous studies that have investigated hippocampal PARV-ir neurons after experimental TBI. Toth et al. (1997) induced TBI in Wistar rats with lateral FPI using mild to moderate impact severity (1.4–2.2 atm) and followed them for up to 8 months. Using stereological analysis they found that 33 % of PARV-ir neurons were remaining in the ipsilateral hilus at 7 days post-TBI. Zhang et al. (2011a, b) also induced mild to moderate (1.8–2.0 atm) lateral FPI in adult Wistar rats. At 7 days post-TBI, they found that 52–55 % of PARV-ir neurons were remaining in the ipsilateral dentate gyrus and 54–56 % in the CA3, whereas the CA1 was intact. The present study extends the previous observations by showing that lateral FPI-induced TBI causes remarkable progressive damage to PARV-ir neurons not only in the dentate gyrus but also in different subfields of the hippocampus proper bilaterally.
Based on our previous experience, we adjusted the SE model to trigger epileptogenesis in about 50 % of the rats by administering diazepam at 2 h after the initiation of SE (Pitkänen et al. 2005). As expected, 39 % of the rats had spontaneous seizures at 6 months post-TBI. Like in the TBI model, the hippocampus is active during spontaneous seizures (Nissinen et al. 2000). From all hippocampal areas investigated, we found a mild reduction in the PARV-ir neurons only in the ipsilateral CA1 at 6 months post-SE, particularly in the pyramidal cell layer, in which 70 % of PARV-ir neurons were remaining. Interestingly, the number of PARV-ir neurons was reduced only in animals that had developed epilepsy by 6 months after SE but not in the non-epilepsy group. Still, there was no correlation between the loss of PARV-ir neurons and seizure frequency. The reduction in neuronal somata was associated with a patchy disappearance of immunopositive axons, particularly septally.
Our studies extend the previous quantitative studies that have demonstrated a reduction in PARV-ir neurons after SE induced by kainate (Buckmaster and Dudek 1997; Kuruba et al. 2011), pilocarpine (André et al. 2001; Dinocourt et al. 2003; Ferrari et al. 2008; Long et al. 2011) or hippocampal stimulation (Gorter et al. 2001; van Vliet et al. 2004; Sun et al. 2007). In all of these models, hippocampal damage develops within hours to days (André et al. 2001; van Vliet et al. 2004; Sun et al. 2007). Buckmaster and Dudek (1997) induced SE with intraperitoneal injections of kainate in adult Sprague–Dawley rats and followed-up the animals for up to 335 days. They counted PARV-ir neurons in the dentate gyrus using an optical fractionator method and found that 78 % of PARV-ir neurons were remaining as compared to controls. Kuruba et al. (2011) also induced SE with intraperitoneal kainate. However, they used adult Fisher 344 rats and perfused the animals for cell counting using an optical fractionator method already at 12 days post-SE. Interestingly, in this rat strain, the reduction of PARV-ir neurons was more robust, resulting in 46 % of neurons remaining in the dentate gyrus, 37 % in the CA3, and 40 % in the CA1. Four studies have quantitatively assessed PARV-ir neurons after pilocarpine-induced SE. André et al. (2001) triggered SE with subcutaneous injections of pilocarpine in adult Sprague–Dawley rats and followed the animals for up to 18 days. Counting of cells from 2 to 3 sections using a 1 cm2 grid revealed that only 20 % of PARV-ir neurons were remaining in the stratum oriens of the CA1, 40 % in the hilus, and 54 % in the granule cell layer of the dentate gyrus. Interestingly, remarkable reductions in neuronal numbers were seen already at 24 h after SE in the stratum oriens of the CA1, that is, before epilepsy onset, progressing towards more chronic time points. In an elegant study by Dinocourt et al. (2003), researchers induced SE with intraperitoneal pilocarpine in adult Wistar rats and followed-up the animals for 3 months. Stereological analysis of the CA1 revealed that 43 % of PARV-ir neurons were remaining in the stratum oriens, but no change was seen in the pyramidal cell layer or stratum radiatum. Ferrari et al. (2008) induced SE with intraperitoneal pilocarpine in Wistar rats and followed-up the animals for 2 months. They found fewer PARV-ir neurons in the CA3 region and in the hilus, but not in the CA1 as compared to controls. Also, Long et al. (2011) induced SE with intraperitoneal injections of pilocarpine in adult Sprague–Dawley rats. They followed the animals for up to 2 months and quantified the neuronal numbers by MetaMorph imaging software from four sections per animal. Analysis revealed that at 1 or 7 days post-SE, there was no change in the number of PARV-ir neurons in hilus, CA3 or CA1. At 15 days post-SE, 88 % of PARV-ir neurons were remaining in the hilus. However, there was a 39 % increase in the number of PARV-ir neurons in the CA1. At 1 and 2 months post-SE, 63 and 58 % of the neurons were remaining in the hilus, respectively. Still, the number of PARV-ir neurons remained increased by 21–46 % in the hippocampus proper. Three studies have quantified PARV-ir neurons after inducing SE with electrical stimulation of the hippocampus or its afferent pathways. Gorter et al. (2001) induced SE in adult Sprague–Dawley rats, followed-up the animals for up to 3 or 6 months and counted cell densities (cells/mm2). They found that 8–16 % of the PARV-ir neurons were remaining in the hilus and 43–44 % in the granule cell layer. Using the same model and counting method, van Vliet et al. (2004) found that after 1 week <30 % of PARV-ir neurons were remaining in the dentate gyrus and 50–60 % in the hippocampus proper. They also reported that in the rats with progressive epilepsy, the number of PARV-ir neurons was comparable to that at 1 week post-SE. However, in a subgroup of rats with non-progressive epilepsy, the number of PARV-ir neurons was comparable to that in controls. Sun et al. (2007) induced SE in adult Sprague–Dawley rats with hippocampal stimulation. They killed rats at different post-SE intervals and one group was followed until they developed spontaneous seizures. They found a reduction in the number of hilar PARV-ir neurons at 1, 3 and 7 days post-SE. However, rats with spontaneous seizures had PARV-ir neuronal numbers comparable to those in controls.
Taken together, even though the methods used to estimate the PARV-ir neuronal numbers vary and some of those methods may cause bias to the cell counts obtained, the data available reveal model-dependency in the reduction of hippocampal PARV-ir neurons after SE and TBI. This can relate to the severity of SE induced by different methods (chemical vs. electrical) or the severity of TBI (moderate vs. severe lateral FPI). Also, the genetic background of the rats and the localization of the primary injury site (hippocampal vs. extrahippocampal) can apparently influence the severity of damage to PARV-ir neurons. The cross-sectional data also suggest that a decline in neuronal numbers and its regional extent is progressive. Perhaps the most striking finding was that the loss of PARV-ir neurons at 6 months after severe TBI was more robust than that in any of the SE models when neuronal numbers had been estimated using unbiased stereology. Yet the rats with TBI did not show spontaneous seizures like the animals subjected to SE. Whether PARV-ir neurons possess a particular sensitivity to the primary mechanical impact associated with TBI remains to be investigated.
Cholecystokinin-immunoreactive interneurons
Unlike PARV-ir neurons, CCK-ir neurons were substantially better preserved both after TBI and SE. At 1 month post-TBI, CCK-ir neuronal numbers were comparable to those of controls. Also at 6 months post-TBI, CCK-positive cells were well preserved except in the dentate gyrus where only about 60 % of CCK neurons remained. Still, however, there was a dense pericellular axonal plexus around the granule cells. Toth et al. (1997) reported that 44 % of hilar CCK-ir neurons were remaining at 1 week after moderate lateral FPI. Zhang et al. (2011a) found that 66 % of CCK-ir neurons were remaining in the dentate gyrus and 60 % in the CA3, whereas the CA1 had normal CCK-ir cell counts at 1 week after mild to moderate lateral FPI. Another study by Zhang et al. (2011b) revealed similar results, that is, 52 % of neurons were remaining in the dentate gyrus, 54 % in the CA3, and no change in the CA1 subfield at 1 week after.
After SE, the total neuronal numbers in different hippocampal subfields remained normal as compared to controls during the 6 months of follow-up. In line with our observations, Buckmaster and Dudek (1997) did not find any change in the total number of CCK-ir neurons in chronically monitored animals with epilepsy after systemic kainate-induced SE. A study by Sun et al. (2007) revealed that there was a loss of CCK-ir neurons in the hilus at 3 and 7 days after electrically induced SE. However, after the rats had become epileptic, the number of hilar CCK-ir neurons was similar to that in controls. Also, they did not find any other signs of neurodegeneration.
Taken together, the little quantitative data available on CCK-ir neurons after epileptogenic injuries suggest that CCK-ir neurons remain better preserved than PARV-ir neurons both after TBI and SE. Interestingly, perisomatic inhibitory neurons, including both PARV-ir and CCK-ir neurons appear more vulnerable to TBI than to SE.
Loss of interneurons responsible of dendritic inhibition after SE and TBI
Calretinin-immunoreactive interneurons
In the rat hippocampus, CR-containing neurons selectively terminate on other interneurons and they are also responsible for the inhibitory control of principal cell dendrites (Freund and Buzsáki 1996; Gulyás et al. 1996). So far, we have not been able to find any previous data on CR-ir neurons after experimental TBI. Here, we found that at 1 month post-TBI, 77 % of CR-ir neurons were remaining in the ipsilateral dentate gyrus. By 6 months, the loss of CR-ir neurons had become bilateral with 74 % remaining ipsilaterally and 77 % contralaterally. Milder reductions in CR-ir neuronal numbers were also found in the CA3.
Unlike after TBI, we did not observe any remarkable changes in the number of CR-ir neurons after SE. Two previous studies have, however, investigated CR-ir neurons after triggering epileptogenesis with pilocarpine-induced SE. André et al. (2001) reported a reduction in CR-ir neurons in all hippocampal subfields of Sprague–Dawley rats, being evident already at 24 h post-SE, and most severe in the stratum oriens of the CA1 with only 20 % of the immunopositive neurons remaining. Interestingly, there was no remarkable progression in the degeneration of CR-ir neurons, not even in animals with spontaneous seizures during the 18-day follow-up. Ferrari et al. (2008) triggered epileptogenesis with intraperitoneal injections of pilocarpine in adult Wistar rats. Animals were killed at 2 months and neuronal numbers were counted from three sections. Like in the study of André et al. (2001), about 30 % of CR-ir neurons were remaining in different hippocampal subfields. van Vliet et al. (2004) induced epileptogenesis with angular bundle stimulation and found that at 1 week post-SE only 35–70 % of CR-ir neurons were remaining in the dentate gyrus, CA3 and CA1 subfields. No further progression in the degeneration of CR-ir neurons was observed by 3–4.5 months post-SE.
Taken together, the little quantitative data available on CR-ir neurons after epileptogenic brain insults suggest that damage to CR-ir neurons increases in the following order: SE induced with electrical amygdala stimulation <TBI induced with lateral FPI < SE induced with electrical hippocampal stimulation <SE induced with systemic pilocarpine. One interpretation is that the remarkable CR-ir neuronal death after systemic pilocarpine and electrical hippocampal stimulation relates to acute SE-induced hippocampal excitotoxicity. After amygdala stimulation-induced SE and lateral FPI, the CR-ir neurons remain better preserved as the acute hippocampal involvement in these models is apparently milder.
Somatostatin-immunoreactive interneurons
TBI caused a remarkable reduction in SOM-ir neuronal numbers. Already at 1 month post-TBI, only 46 % of SOM-ir neurons were remaining in the dentate gyrus ipsilaterally. By 6 months post-TBI, the severity of injury in the dentate gyrus remained similar to that at 1 month. However, the loss of SOM-ir neurons had expanded to the ipsilateral CA3, with 64 % of neurons remaining, as well as to the CA1 with 75 % of SOM-ir neurons remaining. At 6 months, there was also a decrease in SOM-ir neurons in the contralateral CA1 with 78 % of neurons remaining. How these data in the lateral FPI model compare to other models of TBI remains to be explored as currently there are no other data available.
After SE, we found only <15 % reductions in the number of hippocampal SOM-ir neurons during the 6 months of follow-up, which is very mild as compared to previous studies on SOM-ir neurons after SE. Sperk et al. (1992) induced SE with intraperitoneal kainate in Sprague–Dawley rats and followed-up their animals for up to 90 days. They found that already at 2 days post-SE only 20 % of hilar SOM-ir neurons were remaining in the hilus and no further change was found at later follow-up points. Also, Buckmaster and Dudek (1997) induced SE with intraperitoneal kainate in Sprague–Dawley rats. After 66–335 days post-SE, 50 % of SOM-ir neurons were remaining in the dentate gyrus as compared to controls. Using the same model, Buckmaster and Jongen-Rêlo (1999) followed rats for up to 12 months. Unbiased stereological cell counting revealed that 63 % of SOM-ir neurons were remaining in the hilus. Dinocourt et al. (2003) triggered SE in adult Wistar rats with systemic pilocarpine, followed the rats for 3 months post-SE, estimated the neuronal numbers with unbiased stereology in stratum oriens of the CA1, and found that 59 % of SOM-ir neurons were remaining. Also, Long et al. (2011) triggered SE with intraperitoneal pilocarpine but used Sprague–Dawley rats. They followed-up their animals for up to 2 months. Already at 1 day post-SE, only 54 % of SOM-ir neurons were remaining in the hilus, 26 % in the CA3, and 47 % in the CA1. They found, however, that at 2 months post-SE, the number of SOM-ir neurons was actually abnormally high in the CA1 (145 % as compared to controls), but remained reduced in the hilus and CA3 (65–71 % of neurons remaining). Two studies have investigated SOM-ir neurons after hippocampal electrical stimulation induced SE. Gorter et al. (2001) induced SE in adult Sprague–Dawley rats and followed the animals for up to 6 months. The number of hilar SOM-ir neurons in rats with progressive epilepsy was reduced bilaterally, being only 27–28 % of that in controls. In animals with non-progressive epilepsy, 29 % of SOM-ir neurons were remaining ipsilaterally and 68 % contralaterally. Sun et al. (2007) induced SE with hippocampal stimulation and followed the rats for up to 7 days and until some of them had developed recurrent spontaneous seizures. At 1–7 days post-SE, 62–71 % of the hilar SOM-ir neurons were remaining. At later time points in animals that had developed epilepsy, 88 % of SOM-ir neurons remained. Double-staining of Fluoro-Jade B and SOM confirmed the neurodegeneration of immunoreactive neurons.
Taken together, like PARV-ir neurons, SOM-ir neurons are also highly vulnerable to lateral FPI-induced TBI in different hippocampal subfields, particularly in the dentate gyrus. The secondary injury progressing over subsequent months induces further damage and also extends contralaterally. After SE, however, the vulnerability of hippocampal SOM-ir neurons appears to be highly model-dependent.
Neuropeptide Y-immunoreactive interneurons
NPY is the most abundant neuropeptide in the brain. NPY expression is induced by SE and even by spontaneous seizures, and it has shown potent antiepileptic efficacy in experimental models (Vezzani and Sperk 2004; Noè et al. 2008). We found that at 1 month post-TBI, 59 % of NPY-ir neurons were remaining in the ipsilateral dentate gyrus. By 6 months post-TBI, there was no remarkable further reduction in the neuronal numbers in the dentate gyrus where 51 % of NPY-ir neurons were remaining. However, at this chronic time point, the reduction in immunopositive neurons had expanded to the ipsilateral CA3 where only 76 % of NPY-ir neurons were remaining. No changes were observed in the CA1. How these data in the lateral FPI model compare with other models of TBI remains to be explored as currently no other data are available.
Unlike after TBI, we could not perform stereological counting of NPY-ir neurons at 1 month post-SE as the intensive terminal immunoreactivity in the hilus and around the CA1 pyramidal cells compromised the reliability of counting in 29 % of the rats. Analysis of sections sampled at 6 months post-SE revealed a robust bilateral decrease in NPY-ir neurons in rats with epilepsy, but not in non-epileptic rats, as compared to controls. In particular, only 33 % of neurons were remaining in the dentate gyrus ipsilaterally and 37 % contralaterally. In the CA3, 54 % were remaining ipsilaterally and 57 % contralaterally whereas in the CA1 neuronal numbers were normal. We were able to identify four previous studies with quantitative analysis of NPY-ir neuronal numbers after SE. Sperk et al. (1992) induced SE with intraperitoneal kainate in Sprague–Dawley rats and followed these animals for up to 90 days. They found that at 24 h post-SE there was no change in the number of NPY-ir neurons as compared to controls. However, at 2 days post-SE only 39 %, and at 10–90 days only 59 % of NPY-ir neurons were remaining as compared to controls. Kuruba et al. (2011) induced SE with intraperitoneal injection of kainic acid in Fisher 344 rats and assessed neuronal numbers at 12 days post-SE. They reported that 37 % of NPY-ir neurons were remaining in the dentate gyrus and 64 % in the CA3. They did not find any change in the CA1. Long et al. (2011) triggered SE with intraperitoneal pilocarpine in Sprague–Dawley rats and followed the animals for up to 2 months. About 47–77 % of hilar NPY-ir neurons were remaining in different time points in the hilus. At 7 days post-SE, but not at later time points, they noticed a mild decrease in NPY-ir neurons in the CA3 as 87 % of neurons were remaining as compared to controls. A study by Sun et al. (2007) induced SE with hippocampal stimulation and followed rats until they had developed epilepsy. At 3 days post-SE, 67 %, and at 7 days only 36 % of hilar NPY-ir neurons were remaining. Animals with epilepsy had 53 % of their NPY-ir neurons left and neurodegeneration of immunopositive cells was confirmed by double-staining with Fluoro-Jade B and NPY. Thus, unlike with many other interneuron types, the severity of damage to NPY-ir neurons seemed to be similar rather than different after systemic kainate and electrical amygdala stimulation-induced SE.
Taken together, the loss of NPY-ir neurons is robust ipsilaterally after TBI and bilaterally after SE, even when the SE was triggered by unilateral amygdala stimulation. Still, however, we did not find any remarkable correlations between the numbers of NPY-ir neurons and seizure susceptibility or seizure frequency.
Functional implications
In both the TBI and SE model, degeneration of many interneuron types, including PARV-ir and NPY-ir neurons, was progressive, increasing by magnitude over time, becoming more widespread ipsilaterally, and/or extending contralaterally between 1 and 6 months as well. These observations are well in line with our previous magnetic resonance imaging (MRI) studies showing that ipsilateral to TBI, hippocampal abnormalities progressed for about 3 months. Contralaterally, the MRI signal abnormalities were milder than ipsilaterally but also progressed for around 3 months (Immonen et al. 2009). Similarly, in the amygdala stimulation model of SE, the hippocampal signal abnormalities were milder than those seen after TBI but remained for several weeks (Nairismägi et al. 2004). These data suggest that progressive loss of interneurons and their processes contribute to progressive signal abnormality and hippocampal atrophy both after TBI and SE.
Several laboratories have demonstrated a loss of interneurons innervating perisomatic or dendritic compartments of the principal cells in patients with drug-refractory temporal lobe epilepsy (de Lanerolle et al. 1989; Mathern et al. 1995; Maglóczky et al. 2000; Andrioli and Alonso-Nanclares 2007; Tóth et al. 2010). Studies in SE models have extended these reports by demonstrating an association between the reduction in the number of hippocampal GABAergic interneurons and the hyperexcitability or the severity of epilepsy. For example, the elegant study of Buckmaster and Dudek (1997) reported that in the systemic kainate model, rats with the most severe reduction in the number of hilar PARV-ir and CCK-ir neurons had the most remarkable excitability in the dentate gyrus, even though there was no association between the total number of hilar neurons and the frequency of spontaneous seizures. Gorter et al. (2001) showed a connection between the loss of hilar PARV-ir neurons and progression in the severity of epilepsy after electrical SE. One caveat using SE models in such analyses is the co-occurring pathology within extrahippocampal areas, which can be even more important for epileptogenesis than hippocampal network reorganization.
Even though we report a remarkable loss of interneurons at 6 months post-TBI, we were able to find only very few correlations between the severity of interneuron loss and increased seizure susceptibility. Moreover, many of the correlations in the TBI group were towards the opposite direction. Even more surprising was the finding that the rats with TBI had no spontaneous seizures during the 2-week 24/7 video-EEG monitoring period, while the animals in the SE epilepsy group with substantially less reduced interneuron numbers had frequent spontaneous seizures. Thus, the present unbiased quantification of different interneuron classes in well-phenotyped animals challenges the previous idea that the more severe the loss of hippocampal interneurons, the more severe was the epilepsy.
References
André V, Marescaux C, Nehlig A, Fritschy JM (2001) Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus 11:452–468
Andrioli A, Alonso-Nanclares L, Arellano JI, DeFelipe J (2007) Quantative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience 149:131–143
Arellano JI, Muñoz A, Ballesteros-Yáñez I, Sola RG, DeFelipe J (2004) Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain 127:45–64
Bausch SB (2005) Axonal sprouting of GABAergic interneurons in temporal lobe epilepsy. Epilepsy Behav 7:390–400
Bering R, Draguhn A, Diemer NH, Johansen FF (1997) Ischemia changes the coexpression of somatostatin and neuropeptide Y in hippocampal interneurons. Exp Brain Res 115:423–429
Biagini G, Panuccio G, Avoli M (2010) Neurosteroids and epilepsy. Curr Opin Neurol 23:170–176
Blanco MM, dos Santos JG, Jr Perez-Mendes P, Kohek SR, Cavarsan CF, Hummel M, Albuquerque C, Mello LE (2009) Assesment of seizure susceptibility in pilocarpine epileptic and nonepileptic wistar rats and of seizure reinduction with pentylenetetrazole and electroshock models. Epilepsia 50:824–831
Bolkvadze T, Pitkänen A (2012) Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma 29:789–812
Buckmaster PS, Dudek FE (1997) Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 385:385–404
Buckmaster PS, Jongen-Rêlo AL (1999) Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainite-induced epileptic rats. J Neurosci 19:9519–9529
Czuczwar AJ, Patsalos PN (2001) The new generation of GABA enhancers. Potential in the treatment of epilepsy. CNS Drugs 15:339–350
de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD (1989) Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 495:387–395
DeFelipe J (1999) Chandelier cells and epilepsy. Brain 122:1807–1822
Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M (2003) Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol 459:407–425
Ferrari D, Cysneiros RM, Scorza CA, Arida RM, Cavalheiro EA, de Almeida A-CG, Scorza FA (2008) Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav 13:36–42
Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470
Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH (2001) Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci 13:657–669
Gulyás AI, Hájos N, Freund TF (1996) Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci 16:3397–3411
Härtig W, Brauer K, Brückner G (1992) Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. NeuroReport 3:869–872
Immonen RJ, Kharatishvili I, Niskanen J-P, Gröhn H, Pitkänen A, Gröhn OHJ (2009) Distinct MRI pattern in lesional and perilesional area after traumatic brain injury in rat—11 months follow-up. Exp Neurol 215:29–40
Karetko-Sysa M, Skangiel-Kramska J, Nowicka D (2011) Disturbance of perineuronal nets in the perislesional area after photothrombosis is not associated with neuronal death. Exp Neurol 231:113–126
Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A (2006) A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury. Neuroscience 140:685–697
Kharatishvili I, Immonen R, Gröhn O, Pitkänen A (2007) Quantitative diffusion MRI of hippocampus as a surrpgate marker for post-traumatic epileptogenesis. Brain 130:3155–3168
Klausberger T, Somogyi P (2008) Neuronal diversity and temporal dynamics; the unity of hippocampal circuit operations. Science 321:53–57
Kuruba R, Hattiangady B, Parihar VK, Shuai B, Shetty AK (2011) Differential susceptibility of interneurons expressing neuropeptide Y and parvalbumin in aged hippocampus to acute seizure activity. PLoS ONE 6:e24493. doi:10.1371/journal.pone.0024493
Lewis DA, Campbell MJ, Morrison JH (1986) An immonohistochemical characterization of somatostatin-28 and somatostatin-281-12 in monkey prefrontal cortex. J Comp Neurol 248:1–18
Long L, Xiao B, Feng L, Fang Y, Li G, Li A, Mutasem MA, Chen S, Bi F, Li Y (2011) Selective loss and axonal sprouting of GABAergic interneurons in the sclerotic hippocampus induced by LiCI-pilocarpine. Int J Neurosci 121:69–85
Löscher W, Nolting B (1991) The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. IV. Protective indices. Epilepsy Res 9:1–10
Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK (1992) Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci 12:4846–4853
Maglóczky Z (2010) Sprouting in human temporal lobe epilepsy: excitatory pathways and axons of interneurons. Epilepsy Res 89:52–59
Maglóczky Z, Freund TF (2005) Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci 28:334–340
Maglóczky ZS, Wittner L, Borhegyi ZS, Halász P, Vajda J, Czirják S, Freund TF (2000) Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience 96:7–25
Maisano X, Litvina E, Tagliatela S, Aaron GB, Grabel LB, Naegele JR (2012) Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci 32:46–61
Mathern GW, Babb TL, Pretorius JK, Leite JP (1995) Reactive synaptogenesis and neuron densities for neuropeptide y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentate. J Neurosci 15:3990–4004
Mathern GW, Babb TL, Leite JP, Pretorius JK, Yeoman KM, Kuhlman PA (1996) The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res 26:151–161
Mathern GW, Bertram EH, Babb TL, Pretorius JK, Kuhlman PA, Spradlin S, Mendoza D (1997) In contrast to kindled seizures, the frequency of spontaneous epilepsy in the limpic status model correlates with greater aberrant fascia dentate, excitatory and inhibitory axon sprouting, and increased staining for N-Methyl-D-Aspartate, AMPA and GABAA receptors. Neuroscience 77:1003–1019
McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soarest H, Faden AL (1989) Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28:233–244
Nairismägi J, Gröhn OHJ, Kettunen MI, Nissinen J, Kauppinen RA, Pitkänen A (2004) Progression of brain damage after status epilepticus and its association with epileptogenesis: a quantitative MRI study in a rat model of temporal lobe epilepsy. Epilepsia 45:1024–1034
Nissinen J, Halonen T, Koivisto E, Pitkänen A (2000) A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res 38:177–205
Noè F, Pool A-H, Nissinen J, Gobbi M, Bland R, Rizzi M, Balducci C, Ferraguti F, Sperk G, During MJ, Pitkänen A, Vezzani A (2008) Neuropeptide Y gene therapy decreases chronic spontaneous seizures in a rat model of temporal lobe epilepsy. Brain 131:1506–1515
Ojemann GA (1987) Surgical therapy for medically intractable epilepsy. J Neurosurg 66:489–499
Pavlov I, Huusko N, Drexel M, Kirchmair E, Sperk G, Pitkänen A, Walker MC (2011) Progressive loss of phasic, but not tonic GABAA receptor mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience 194:208–219
Paxinos G, Watson C (1986) The Rat Brain in Stereotaxic Coordinates. Academic Press, New York
Pitkänen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10:173–186
Pitkänen A, Sutula TP (2002) Is epilepsy progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancer Neurol 1:173–181
Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J (2005) Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res 63:27–42
Rattka M, Brandt C, Bankstahl M, Bröer S, Löscher W (2011) Enhanced susceptibility to the GABA antagonist pentylenetetrazole during the latent period following a pilpcarpine-induced status epilepticus in rats. Neuropharmacology 60:505–512
Schmued LC, Hopkins KJ (2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874:123–130
Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, Potier MC, Celio MR, Villa AEP (2004) Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci 25:650–663
Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G (1995) Somatostatin, neuropeptide y, neurokinin b and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience 69:831–845
Sloviter RS (1991) Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1:41–66
Sloviter RS, Ali-Akbarian L, Horvart KD, Menkens KA (2001) Substance p receptor expression by inhibitory interneurons of the rat hippocampus: enhanced detection using improved immunocytochemical methods for the preservation and colocalization of GABA and other neuronal markers. J Comp Neurol 430:283–305
Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M (2003) “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepsticus in the rat. J Comp neurol 459:44–76
Söderpalm B (2002) Anticonvulsants: aspects of their mechanisms of action. Eur J Pain 6:3–9
Sperk G, Marksteiner J, Bellmann GR, Mahata M, Ortler M (1992) Functional changes in neuropeptide Y- and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience 50:831–846
Sun C, Mtchedlishvili Z, Bertram AE, Kapur J (2007) Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol 500:876–893
Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS (2010) Initial loss but later excess of GABAergic synapses with granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol 518:647–667
Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK (2005) Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma 22:42–75
Toth Z, Hollrigel GS, Gorcs T, Soltesz I (1997) Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci 17:8106–8117
Tóth K, Erőss L, Vajda J, Halász P, Freund TF, Maglóczky Z (2010) Loss and reorganization of calretinin-containing interneurons in the epileptic human hippocampus. Brain 133:2763–2777
van Vliet EA, Aronica E, Tolner EA, Lopes da Silva FH, Gorter JA (2004) Progression of temporal lobe epilepsy in the rat is associated with immunocytochemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp Neurol 187:367–379
Vezzani A, Sperk G (2004) Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides 38:245–252
Vreugdenhil M, Jefferys JGR, Celio MR, Schwaller B (2003) Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol 89:1414–1422
Wasterlain CG, Shirasaka Mazarati AM, Spigelman I (1996) Chronic epilepsy with damage restricted to the hippocampus: possible mechanisms. Epilepsy Res 26:255–265
West MJ, Slomianka L, Gundersen HJG (1991) Unbiased stereological estimation of the total number of neurons in the subvisions of the rat hippocampus using the optical fractionators. Anat Rec 231:482–497
Wittner L, Maglóczky ZS, Borhegyi ZS, Halász P, Tóth SZ, Erőss L, Szabó Z, Freund TF (2001) Preservation of perisomatic inhibitory input of granule cells in the epileptic human hippocampus. Neuroscience 108:587–600
Zhang N, Wei W, Mody I, Houser CR (2007) Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci 27:7520–7531
Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince D, Buckmaster P (2009) Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci 29:14247–14256
Zhang B, Chen X, Lin Y, Tan T, Yang Z, Dayao C, Liu L, Jiang R, Zhang J (2011a) Impairment of synaptic plasticity in hippocampus is exacerbated by methylprednisolone in a rat model of traumatic brain injury. Brain Res 1382:165–172
Zhang B, Chen X, Tan T, Yang Z, Carlos D, Jiang R, Zhang J (2011b) Traumatic brain injury impairs synaptic plasticity in hippocampus in rats. Chin Med J 124:740–745
Acknowledgments
This study was supported by the FP6 Grant LSHM-CT-2006-037315 (A.P.), Academy of Finland (A.P.), The Sigrid Juselius Foundation (A.P.), The North-Savo Regional Fund of The Finnish Cultural Foundation (N.H.), and The Finnish Epilepsy Foundation (N.H.). We thank Mr Jarmo Hartikainen and Mrs Merja Lukkari for their excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
429_2013_644_MOESM8_ESM.pdf
Online Resource 8. Supplementary Figure 1. Representative examples of substance P receptor (SPR) immunoreactivity (ir) in the ipsilateral septal dentate gyrus in three rats. Percentages in panels B and C indicate the magnitude in reduction of PARV immunolabeled neurons in different layers of the dentate gyrus after TBI. (A) A control rat. Arrows indicate numerous SPR-ir somata with immunopositive dendrites in the granule cell and molecular layers, and a dense fiber plexus in the hilus. (B) A rat with TBI 1 month earlier. The animal had 50% of PARV-ir neurons remaining in the ipsilateral dentate gyrus at 1 month post-TBI. Laminar analysis revealed that the rat had 91% (-9%) of PARV-ir neurons remaining in the molecular layer, 71% (-29%) in the granule cell layer, and 56% (-44%) in the hilus as compered to the control mean. Note a reduction in SPR-ir neurons and immunopositive fibers in the infragranular region and the hilus, respectively, as compared to the control rat (panel A). Arrows indicate some of the remaining SPR-ir somata. (C) A rat with TBI 6 months earlier. The animal had 35% of PARV-ir neurons remaining in the ipsilateral dentate gyrus at 6 months post-TBI. Laminar analysis revealed that the rat had 79% (-21%) of PARV-ir neurons remaining in the molecular layer, 36% (-64%) in the granule cell layer and 18% (-88%) in the hilus as compered to the control mean. Arrows indicate two remaining SPR-ir somata. Abbreviations: GCL, granule cell layer; H, hilus; MOL, molecular layer; TBI, traumatic brain injury. Scale bar equal 50 µm in all panels. (PDF 423 kb)
Rights and permissions
About this article
Cite this article
Huusko, N., Römer, C., Ndode-Ekane, X.E. et al. Loss of hippocampal interneurons and epileptogenesis: a comparison of two animal models of acquired epilepsy. Brain Struct Funct 220, 153–191 (2015). https://doi.org/10.1007/s00429-013-0644-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0644-1