Abstract
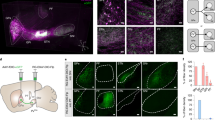
The tegmental pedunculopontine nucleus (PPN) is a basal ganglia-related structure that has recently gained renewed interest as a potential surgical target for the treatment of several aspects of Parkinson’s disease. However, the underlying anatomical substrates sustaining the choice of the PPN nucleus as a surgical candidate remain poorly understood. Here, we characterized the chemical phenotypes of different subtypes of PPN efferent neurons innervating the rat parafascicular (PF) nucleus. Emphasis was placed on elucidating the impact of unilateral nigrostriatal denervation on the expression patterns of the mRNA coding the vesicular glutamate transporter type 2 (vGlut2 mRNA). We found a bilateral projection from the PPN nucleus to the PF nucleus arising from cholinergic and glutamatergic efferent neurons, with a small fraction of projection neurons co-expressing both cholinergic and glutamatergic markers. Furthermore, the unilateral nigrostriatal depletion induced a bilateral twofold increase in the expression levels of vGlut2 mRNA within the PPN nucleus. Our results support the view that heterogeneous chemical profiles account for PPN efferent neurons innervating thalamic targets. Moreover, a bilateral enhancement of glutamatergic transmission arising from the PPN nucleus occurs following unilateral dopaminergic denervation, therefore sustaining the well-known hyperactivity of the PF nucleus in parkinsonian-like conditions. In conclusion, our data suggest that the ascending projections from the PPN that reach basal ganglia-related targets could play an important role in the pathophysiology of Parkinson’s disease.





Similar content being viewed by others
References
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Aymerich MS, Barroso-Chinea P, Pérez-Manso M, Muñoz-Patiño AM, Moreno-Igoa M, González-Hernández T, Lanciego JL (2006) Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci 23:2009–2108
Aziz TZ, Davies L, Stein J, France S (1998) The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br J Neurosurg 12:245–249
Bacci JJ, Kachidian P, Kerkerian-Le Goff L, Salin P (2004) Intralaminar thalamic nuclei lesions: widespread impact on dopamine denervation-mediated cellular defects in the rat basal ganglia. J Neuropathol Exp Neurol 63:20–31
Blanco-Lezcano L, Rocha-Arrieta LL, Alvarez-González L, Martínez-Martí L, Pavón-Fuentes N, González-Fraguela ME, Bauzá-Calderín Y, Coro-Grave de Peralta Y (2005) The effects of lesions in the compact parto f the substantia nigra on glutamate and GABA release in the pedunculopontine nucleus. Rev Neurol 40:23–29
Breit S, Bouali-Benazzouz R, Benabid AL, Benazzouz A (2001) Unilateral lesion of the nigrostriatal pathway induces an increase on neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur J Neurosci 14:1833–1842
Breit S, Lessmann L, Unterbrink D, Popa RC, Gasser T, Schulz JB (2006) Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur J Neurosci 24:2275–2282
Breit S, Martin A, Lessmann L, Cerkez D, Gasser T, Schulz JB (2008) Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J Neurosci Res 86:1388–1396
Caparrós-Lefebvre D, Ruchoux MM, Blond D, Petit H, Percheron G (1994) Long-term thalamic stimulation in Parkinson’s disease: postmortem anatomoclinical study. Neurology 44:1856–1860
Caparrós-Lefebvre D, Blond S, Feltin MP, Pollak P, Benabid AL (1999) Improvement of levodopa induced dyskinesias by thalamic deep brain stimulation is related to slight variations in electrode placement: possible involvement of the centre median parafascicularis complex. J Neurol Neurosurg Pshychiatr 67:308–314
Capozzo A, Florio T, Cellini R, Moriconi U, Scarnati E (2003) The pedunculopontine nucleus projectin to the parafascicular nucleus of the thalamus: an electrophysiological investigation in the rat. J Neural Transm 110:733–747
Carlson JD, Pearlstein RD, Bucholz J, Iacono RP, Maoda G (1999) Regional metabolic changes in the pedunculopontine nucleus of unilateral 6-hydroxydopamine Parkinson’s model rats. Brain Res 828:12–19
Clements JR, Grant S (1990) Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett 120:70–73
Cornwall J, Phillipson OT (1988) Afferent projections to the parafascicular thalamic nucleus of the rat, as shown by the retrograde transport of wheat germ agglutinin. Brain Res Bull 20:139–150
Crossman AR (1987) Primate models of dyskinesia: the experimental approach in the study of basal ganglia-related involuntary movement disorders. Neuroscience 21:1–40
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:266–271
Dun NJ, Dun SL, Hwang LL, Fostermann U (1995) Infrequent co-existence of nitric oxide synthase and parvalbumin, calbindin and calretinin immunoreactivity in rat pontine neurons. Neurosci Lett 191:165–168
Erro E, Lanciego JL, Giménez-Amaya JM (1999) Relationships between thalamostriatal neurons and pedunculopontine projections to the thalamus: a neuroanatomical tract-tracing study in the rat. Exp Brain Res 127:162–170
Ford B, Holmes CJ, Mainville L, Jones BE (1995) GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol 363:177–196
García-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, Husain M, Karson CN (1995) Mesopontine neurons in schizophrenia. Neuroscience 66:321–335
Geula C, Schatz CR, Mesulam MM (1993) Differential localization of NADPH-diaphorase and calbindin-D28 k within the cholinergic neurons in the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience 54:461–476
Gonzalo N, Lanciego JL, Castle M, Vázquez A, Erro E, Obeso JA (2002) The parafascicular thalamic complex and basal ganglia circuitry: further complexity to the basal ganglia model. Thalamus Relat Sys 1:341–348
Groenewegen HJ, Berendse HW, Haber SN (1993) Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience 57:113–142
Hallanger AE, Wainer BH (1988) Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol 274:483–515
Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH (1987) The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol 262:105–124
Isaacson LG, Tanaka D Jr (1986) Cholinergic and non-cholinergic projections from the canine pontomesencephalic tegmentum (Ch5 area) to the caudal intralaminar nuclei. Exp Brain Res 62:179–188
Jackson A, Crossman AR (1983) Nucleus tegmenti pedunculopontininus: efferent connections with special referente to the basal ganglia, studied in the rat by anterograde and retrograde transporto f horseradish peroxidase. Neuroscience 10:725–765
Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ (2004) Pedunculopontine nucleus stimulation improves akinesia in a parkinsonian monkey. NeuroReport 15:2621–2624
Jenkinson N, Nandi D, Aziz TZ, Stein JF (2005) Pedunculopontine nucleus: a new target for deep brain stimulation for akinesia. NeuroReport 16:1875–1876
Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH (2003) Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a Light and electron microscopic study. Brain Res 992:205–219
Jones EG (1991) Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience 40:637–656
Kobayashi S, Nakamura Y (2003) Synaptic organization of the rat parafascicular nucleus, with special reference to its afferents from the superior colliculus and the pedunculopontine tegmental nucleus. Brain Res 980:80–91
Kobayashi S, Hoshino K, Homma S, Takagi S, Norita M (2007) A possible monosynaptic pathways links the pedunculopontine nucleus to thalamostriatal neurons in the hooded rat. Arch Histol Cytol 70:207–214
Kojima J, Yamaji Y, Matsumara M, Nambu A, Inase M, Tokuno H, Takada M, Imai H (1997) Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralatral hemiparkinsonism in the monkey. Neurosci Lett 226:111–114
Krauss JK, Pohle T, Weigel R, Burgunder JM (2002) Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J Neurol Neurosurg Psychiatr 72:546–548
Krout KE, Belzer RE, Loewy AD (2002) Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 448:53–101
Lanciego JL, Rodríguez-Oroz MC, Blesa FJ, Alvarez-Erviti L, Guridi J, Barroso-Chinea P, Smith Y, Obeso JA (2008) Lesion of the centromedian thalamic nucleus in MPTP-treated primates. Mov Disord 23:708–715
Lavoie B, Parent A (1994) Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol 344:210–231
Martínez-González C, Bolam JP, Mena-Segovia J (2011) Topographical organization of the pedunculopontine nucleus. Frontiers Neuroanat. doi:10.3389/fnana.2011.00022
Mazzone P, Lozano A, Stnzione P, Galati S, Scarnati E, Peppe A, Stefani A (2005) Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. NeuroReport 16:1877–1881
Mena-Segovia J, Micklem BR, Nair-Roberts RG, Ungless MA, Bolam JP (2009) GABAergic neuron distribution in the pedunculopontine nucleus defines functional subterritories. J Comp Neurol 515:397–408
Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: an overview based on alternative nomenclature (Ch1–Ch6). Neuroscience 10:1185–1201
Moriizumi T, Hattori T (1992) Separate neuronal populations of the rat globus pallidus projecting to the subthalamic nucleus, auditory cortex and pedunculopontine tegmental area. Neuroscience 46:701–710
Munro-Davis LE, Winter J, Aziz TZ, Stein JF (1999) The role of the pedunculopontine region in basal-ganglia mechanisms of akinesia. Exp Brain Res 129:511–517
Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF (2002a) Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain 125:2418–2430
Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF (2002b) Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci 9:170–174
Oakman SA, Faris PL, Cozzari C, Hartman BK (1999) Characterization of the extent of pontomesencephalic cholinergic neurons’ projections to the thalamus: comparison with projections to midbrain dopaminergic groups. Neuroscience 94:529–547
Orieux G, François C, Féger J, Yelnik J, Vila M, Ruberg G, Agid Y, Hirsch EC (2000) Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience 97:79–88
Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123:1767–1783
Parent M, Descarries L (2008) Acetylcholine innervation of the adult rat thalamus: distribution and ultrastructural features in dorsolateral geniculate, parafascicular and reticular thalamic nuclei. J Comp Neurol 511:678–691
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Plaha P, Gill SS (2005) Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. NeuroReport 16:1883–1887
Rosene DL, Roy NJ, Davis BJ (1986) A cryoprotection method that facilitates cutting frozen sections of whole monkeys brain for histological and histochemical processing without freezing artifact. J Histochem Cytochem 34:1301–1316
Saper CB, Loewy AD (1982) Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res 252:367–372
Semba K, Fibiger HC (1992) Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol 323:387–410
Semba K, Reiner PB, Fibiger HC (1990) Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38:643–654
Sofroniew MV, Priestley JV, Consolazione A, Eckenstein F, Cuello AC (1985) Cholinergic projections from the midbrain and pons to the thalamus in the rat, identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry. Brain Res 329:213–223
Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P (2007) Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 130:1596–1607
Steininger TL, Rye DB, Wainer BH (1992) Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. J Comp Neurol 321:515–543
Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC (1990) Neuronal activities in brainstem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci 10:2541–2559
Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG (2002) Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and non-aminergic vasomotor neurons of the rat medulla. J Comp Neurol 444:207–220
Sugimoto T, Hattori T (1984) Organization of efferent projections of nucleus tegmenti pedunculopontinus pars compacta with special reference to its cholinergic aspects. Neuroscience 11:931–946
Vila M, Périer C, Féger J, Yelnik J, Faucheux B, Ruberg M, Raisman-Vozari R, Agid Y, Hirsch EC (2000) Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by the metabolic and electrophysiological measurements. Eur J Neurosci 12:337–344
Vincent SR (2000) The ascending reticular activating system: from aminergic neurons to nitric oxide. J Chem Neuroanat 18:23–30
Vincent SR, Satoh K, Armstrong DM, Fibiger HC (1983) NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett 43:31–36
Wang H-L, Morales M (2009) Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29:340–358
Acknowledgments
This study was supported by Departamento de Salud Gobierno de Navarra, Ministerio de Ciencia e Innovación (BFU2009-08351), CIBERNED (CB06/05/0006), FIS (PI051037) and by the UTE project/Foundation for Applied Medical Research (FIMA). Salary for S.S. is partially supported by a grant from Mutual Médica.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barroso-Chinea, P., Rico, A.J., Conte-Perales, L. et al. Glutamatergic and cholinergic pedunculopontine neurons innervate the thalamic parafascicular nucleus in rats: changes following experimental parkinsonism. Brain Struct Funct 216, 319–330 (2011). https://doi.org/10.1007/s00429-011-0317-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-011-0317-x