Abstract
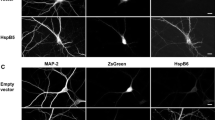
The so-called stress response involving upregulation of heat shock proteins (Hsps) is a powerful mechanism of cells to deal with harmful conditions to which they are exposed throughout life, such as hyperthermia, hypoxia or oxidative stress. To gain more information about the molecular targets by which HspB1 (Hsp25) and HspB5 (αB-crystallin) might exert their neuroprotective effect we investigated the subcellular localization of unphosphorylated and phosphorylated HspB1 and B5 in neurons by immunocytochemistry and subcellular fractionation. In cultured hippocampal neurons, the unphosphorylated forms of both Hsps were localized in the perikaryon and nucleus, whereas the phosphorylated forms were recruited into neuronal processes. pHspB1-Ser15 and -Ser 86 were found within dendrites with a punctate distribution pattern partially colocalizing with the synaptic marker vGlut-1. pHspB5-Ser19 and -Ser45 localized to axons and dendrites with a filamentous-like staining pattern, whereas pHspB5-Ser59 was found in dendrites, especially along the plasma membrane and in spines. Biochemical analysis, i.e. subcellular fractionation of rat brain with subsequent Western blotting supported these localizations. These data show that in neurons HspB1 and B5 may have various molecular interaction partners at synapses, within dendrites and axons and that this interaction is likely to be regulated by phosphorylation. Stress-induced phosphorylation of HspB1 and B5 may lead to binding of these Hsps to their targets at synapses and neuronal processes which might provide one important mechanism of how they exert their neuroprotective effect.





Similar content being viewed by others
References
Ackerley S, James PA, Kalli A, French S, Davies KE, Talbot K (2006) A mutation in the small heat-shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet 152:347–354
Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, Wynn S, Wells DJ, Latchman DS, de Belleroche J (2003) The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem 27822:19956–19965
Almeida-Souza L, Asselbergh B, d’Ydewalle C, Moonens K, Goethals S, de Winter V, Azmi A, Irobi J, Timmermans JP, Gevaert K, Remaut H, Van Den Bosch L, Timmerman V, Janssens S (2011) Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J Neurosci 31(43):15320–15328
Arrigo AP, Welch WJ (1987) Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem 26232:15359–15369
Arrigo AP, Suhan JP, Welch WJ (1988) Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol 812:5059–5071
Barbato R, Menabo R, Dainese P, Carafoli E, Schiaffino S, Di Lisa F (1996) Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ Res 785:821–828
Bartelt-Kirbach B, Golenhofen N (2011) Differential expression and induction of small heat shock proteins in rat brain and cultured hippocampal neurons. J Neurosci Res 892:162–175
Bechtold DA, Brown IR (2000) Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Brain Res Mol Brain Res 752:309–320
Bellyei S, Szigeti A, Pozsgai E, Boronkai A, Gomori E, Hocsak E, Farkas R, Sumegi B, Gallyas F Jr (2007) Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol 863:161–171
Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ (2002) Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 361:45–56
Bryantsev AL, Chechenova MB, Shelden EA (2007) Recruitment of phosphorylated small heat shock protein Hsp27 to nuclear speckles without stress. Exp Cell Res 3131:195–209
Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, Ding L, Labeit S, Horwitz J, Leonard KR, Linke WA (2004) Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem 2799:7917–7924
Carlin RK, Grab DJ, Cohen RS, Siekevitz P (1980) Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol 863:831–845
Charette SJ, Lavoie JN, Lambert H, Landry J (2000) Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 2020:7602–7612
Chiesa R, Gawinowicz-Kolks MA, Kleiman NJ, Spector A (1987) The phosphorylation sites of the B2 chain of bovine alpha-crystallin. Biochem Biophys Res Commun 1443:1340–1347
Chiesi M, Longoni S, Limbruno U (1990) Cardiac alpha-crystallin. III. Involvement during heart ischemia. Mol Cell Biochem 972:129–136
Cobb BA, Petrash JM (2000) Characterization of alpha-crystallin-plasma membrane binding. J Biol Chem 2759:6664–6672
Danan IJ, Rashed ER, Depre C (2007) Therapeutic potential of H11 kinase for the ischemic heart. Cardiovasc Drug Rev 251:14–29
Djabali K, de Nechaud B, Landon F, Portier MM (1997) AlphaB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci 110Pt 21:2759–2769
Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V (2004) Mutant small heat-shock protein 27 causes axonal Charcot–Marie–Tooth disease and distal hereditary motor neuropathy. Nat Genet 366:602–606
Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG (2005) Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation 11114:1792–1799
Gaestel M, Schroder W, Benndorf R, Lippmann C, Buchner K, Hucho F, Erdmann VA, Bielka H (1991) Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J Biol Chem 26622:14721–14724
Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP (2011) AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem 2865:3261–3269
Garrido C, Gurbuxani S, Ravagnan L, Kroemer G (2001) Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 2863:433–442
Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D (1998) Ischemia-induced phosphorylation and translocation of stress protein alpha B-crystallin to Z lines of myocardium. Am J Physiol 2745(Pt 2):H1457–H1464
Golenhofen N, Arbeiter A, Koob R, Drenckhahn D (2002) Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol 343:309–319
Golenhofen N, Perng M, Quinlan RA, Drenckhahn D (2004) Comparison of the small heat shock proteins alpha-B-crystallin, MKBP, HSP25, HSP20 and cvHSP in heart and skeletal muscle. Histochem Cell Biol 122:415–425
Golenhofen N, Redel A, Wawrousek EF, Drenckhahn D (2006) Ischemia-induced increase of stiffness of alphaB-crystallin/HSPB2-deficient myocardium. Pflugers Arch 4514:518–525
Graner MW, Cumming RI, Bigner DD (2007) The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences. J Neurosci 2742:11214–11227
Gusev NB, Bogatcheva NV, Marston SB (2002) Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry (Mosc) 675:511–519
Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 1210:842–846
Heinzel W, Vogt A, Kallee E, Faller W (1965) A new method for the quantitative determination of antibody and antigen protein, with a sensitivity to five micrograms. J Lab Clin Med 66:334–340
Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH (2004) Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation 11023:3544–3552
Ifeanyi F, Takemoto L (1990) Specificity of alpha crystallin binding to the lens membrane. Curr Eye Res 93:259–265
Irobi J, Van Impe K, Seeman P, Jordanova A, Dierick I, Verpoorten N, Michalik A, De Vriendt E, Jacobs A, Van Gerwen V, Vennekens K, Mazanec R, Tournev I, Hilton-Jones D, Talbot K, Kremensky I, Van Den Bosch L, Robberecht W, Van Vandekerckhove J, Van Broeckhoven C, Gettemans J, De Jonghe P, Timmerman V (2004) Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet 366:597–601
Ito H, Okamoto K, Nakayama H, Isobe T, Kato K (1997) Phosphorylation of alphaB-crystallin in response to various types of stress. J Biol Chem 27247:29934–29941
Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K (2001) Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem 2767:5346–5352
Kamei A, Hamaguchi T, Matsuura N, Masuda K (2001) Does post-translational modification influence chaperone-like activity of alpha-crystallin? I. Study on phosphorylation. Biol Pharm Bull 241:96–99
Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW (2003) The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones 81:53–61
Kato K, Hasegawa K, Goto S, Inaguma Y (1994) Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem 26915:11274–11278
Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S (1998) Phosphorylation of alphaB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem 27343:28346–28354
Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K et al (1990) ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res 5281:21–24
Kolb SJ, Snyder PJ, Poi EJ, Renard EA, Bartlett A, Gu S, Sutton S, Arnold WD, Freimer ML, Lawson VH, Kissel JT, Prior TW (2010) Mutant small heat shock protein B3 causes motor neuropathy: utility of a candidate gene approach. Neurology 746:502–506
Kostenko S, Moens U (2009) Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci 6620:3289–3307
Kyhse-Andersen J (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods 103–4:203–209
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227259:680–685
Landry J, Chretien P, Laszlo A, Lambert H (1991) Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol 1471:93–101
Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW (1992) Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 2672:794–803
Liebau S, Proepper C, Schmidt T, Schoen M, Bockmann J, Boeckers TM (2009) ProSAPiP2, a novel postsynaptic density protein that interacts with ProSAP2/Shank3. Biochem Biophys Res Commun 3853:460–465
Loktionova SA, Ilyinskaya OP, Gabai VL, Kabakov AE (1996) Distinct effects of heat shock and ATP depletion on distribution and isoform patterns of human Hsp27 in endothelial cells. FEBS Lett 3922:100–104
McClaren M, Isseroff RR (1994) Dynamic changes in intracellular localization and isoforms of the 27-kD stress protein in human keratinocytes. J Invest Dermatol 1023:375–381
Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC (2003) Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res 922:203–211
Narayanan S, Kamps B, Boelens WC, Reif B (2006) alphaB-crystallin competes with Alzheimer’s disease beta-amyloid peptide for peptide-peptide interactions and induces oxidation of Abeta-Met35. FEBS Lett 58025:5941–5946
Nicholl ID, Quinlan RA (1994) Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J 134:945–953
Obrenovitch TP (2008) Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev 881:211–247
Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L (2007) Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 4487152:474–479
Panasenko OO, Kim MV, Marston SB, Gusev NB (2003) Interaction of the small heat shock protein with molecular mass 25 kDa (hsp25) with actin. Eur J Biochem 2705:892–901
Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA (1999) Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci 112Pt 13:2099–2112
Quraishe S, Asuni A, Boelens WC, O’Connor V, Wyttenbach A (2008) Expression of the small heat shock protein family in the mouse CNS: Differential anatomical and biochemical compartmentalization. Neuroscience 1532:483–491
Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK (2001) Transgene overexpression of aB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. Faseb J 152:393–402
Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW (1994) Expression of alpha B-crystallin in Alzheimer’s disease. Acta Neuropathol 872:155–160
Ritossa FM (1962) A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia 18:571–573
Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR (2010) alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE 510:e12578
Stege GJ, Renkawek K, Overkamp PS, Verschuure P, van Rijk AF, Reijnen-Aalbers A, Boelens WC, Bosman GJ, de Jong WW (1999) The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochem Biophys Res Commun 2621:152–156
Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M (1992) Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett 3133:307–313
Sun Y, MacRae TH (2005) The small heat shock proteins and their role in human disease. FEBS J 27211:2613–2627
van den IJssel P, Wheelock R, Prescott A, Russell P, Quinlan RA (2003) Nuclear speckle localisation of the small heat shock protein alpha B-crystallin and its inhibition by the R120G cardiomyopathy-linked mutation. Exp Cell Res 2872:249–261
van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri M, Polman CH, Lassmann H, Ravid R (1995) The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature 3756534:798–801
van Rijk AE, Stege GJ, Bennink EJ, May A, Bloemendal H (2003) Nuclear staining for the small heat shock protein alphaB-crystallin colocalizes with splicing factor SC35. Eur J Cell Biol 827:361–368
Verschuure P, Croes Y, van den IPR, Quinlan RA, de Jong WW, Boelens WC (2002) Translocation of small heat shock proteins to the actin cytoskeleton upon proteasomal inhibition. J Mol Cell Cardiol 342:117–128
Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC (2003) Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol 8210:523–530
Voorter CE, Wintjes L, Bloemendal H, de Jong WW (1992) Relocalization of alpha B-crystallin by heat shock in ovarian carcinoma cells. FEBS Lett 3092:111–114
Vos MJ, Hageman J, Carra S, Kampinga HH (2008) Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry 4727:7001–7011
Wang K, Spector A (1996) alpha-Crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem 2421:56–66
Wang J, Martin E, Gonzales V, Borchelt DR, Lee MK (2008) Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases. Neurobiol Aging 294:586–597
Welch WJ (1992) Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 724:1063–1081
Wieske M, Benndorf R, Behlke J, Dolling R, Grelle G, Bielka H, Lutsch G (2001) Defined sequence segments of the small heat shock proteins HSP25 and alphaB-crystallin inhibit actin polymerization. Eur J Biochem 2687:2083–2090
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, de Waal RM, Verbeek MM (2006a) Small heat shock protein HspB8: its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol 1112:139–149
Wilhelmus MM, Otte-Holler I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM (2006b) Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer’s disease brains. Neuropathol Appl Neurobiol 322:119–130
Williams KL, Mearow KM (2011) Phosphorylation status of heat shock protein 27 influences neurite growth in adult dorsal root ganglion sensory neurons in vitro. J Neurosci Res 898:1160–1172
Xi JH, Bai F, McGaha R, Andley UP (2006) Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. Faseb J 207:846–857
Acknowledgments
We thank Bianca Mekle and Silke Zschemisch for their excellent technical assistance and the International Graduate School in Molecular Medicine (University of Ulm, Germany) of which Thomas Schmidt is a member.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2012_964_MOESM1_ESM.pdf
Supplementary figure 1: Cultured hippocampal neurons immunolabelled for phospho-HspB1 and phospho-HspB5 as indicated (a-e) without and (f-j) with preabsorption of the antibodies with the respective immunogen. Note strong reduction or absence of immunosignals in panels f-j compared to panels a-e demonstrating specificity of the phospho-specific-Hsp-antibodies. Bar: 20 μm (PDF 558 kb)
418_2012_964_MOESM2_ESM.pdf
Supplementary figure 2: Quantification of band intensities of Western blots for HspB1, B5 and their phospho-forms of subcellular fractionations of rat brains as shown for a representative experiment in figure 5. HspB1 was significantly enriched in the nuclear fraction but also present in all other fractions investigated. pHspB1-Ser86 showed a similar subcellular distribution whereas pHspB1-S15 was enriched in the membrane fraction, synaptosomes and synaptic junctions and was detectable in little amount in the postsynaptic densities. HspB5 was enriched in the myelin, membrane fraction and postsynaptic densities The immunoblots using the phospho-specific antibodies against HspB5 showed that HspB5 in the myelin fraction was strongly phosphorylated at all three phosphorylation sites. Regarding the synaptic fractions pHspB5-Ser 19 was not present in any of these fractions analyzed. pHspB5-Ser45 and -Ser59 were detectable in low amounts in synaptosomes and synaptic membranes and to a much higher amount in postsynaptic densities. This hints to a postsynaptic localization of these two phospho forms of HspB5. n = 3, * p < 0.05 versus homogenate (PDF 109 kb)
Rights and permissions
About this article
Cite this article
Schmidt, T., Bartelt-Kirbach, B. & Golenhofen, N. Phosphorylation-dependent subcellular localization of the small heat shock proteins HspB1/Hsp25 and HspB5/αB-crystallin in cultured hippocampal neurons. Histochem Cell Biol 138, 407–418 (2012). https://doi.org/10.1007/s00418-012-0964-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-0964-x