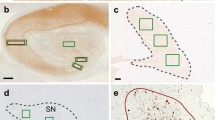
Abstract
Filamentous protein inclusions in neurons (Lewy bodies, LB) and dystrophic neurites containing pathologic α-synuclein (αSyn) are the morphologic hallmarks of sporadic Parkinson disease (PD) and dementia with Lewy bodies (DLB), but are also found in aged subjects and in a variety of neurogenerative disorders. They occur in the central, peripheral, and autonomic nervous system as an essential or coincident feature. Their formation runs through several phases from initial dust-like particles cross-linked with αSyn to aggregation of ubiquitinated dense filaments, formation of LBs, finally degradation and death of the afflicted neurons. Pathologic accumulation of αSyn/LBs proposed by Braak et al. (Neurobiol Aging 24:197–211, 2003), following a predictable sequence of lesions in six stages with ascending progression from medullary and olfactory nuclei to the cortex, has been considered to be linked to clinical dysfunctions. The consensus pathologic guidelines of DLB (Neurology 65:1863–1872, 2005), by semiquantitative scoring to αSyn pathology (LB density and distribution) in specific brain regions, distinguish three phenotypes (brainstem, transitional/limbic, and diffuse neocortical), and also consider concomitant Alzheimer-related pathology. αSyn pathology in the amygdala is often associated with Alzheimer disease. Although some retrospective clinico-pathologic studies have largely confirmed the Braak LB staging system, it shows neither correlation to the clinical severity and duration of parkinsonism nor to nigral αSyn burden and cell loss which significantly correlates with resulting striatal loss of dopamine, dopamine transporter and tyrosine hydroxylase, duration and severity of motor dysfunction. Between 6.3 and 43% of clinically manifested PD cases did not follow this pattern, and in 7–8.3% of those with αSyn-positive inclusions in midbrain and cortex the medullary nuclei were spared. On the other hand, 30–55% of elderly subjects with widespread Lewy pathology revealed no neuropsychiatric symptoms or were not classifiable. Therefore, detection and staging of Lewy pathology without assessment of neuronal loss in specific areas may not have clinical impact and its predictive validity is questionable. For demented patients, modified criteria for categorization of Lewy pathology were proposed. If robust correlations between clinical course and Lewy/αSyn pathology are to be confirmed by future studies, the currently used morphologic staging/classification systems should be revised accordingly.

Similar content being viewed by others
References
Aho L, Parkkinen L, Pirttila T, Alafuzoff I (2008) Systematic appraisal using immunohistochemistry of brain pathology in aged and demented subjects. Dement Geriatr Cogn Disord 25:423–432
Alafuzoff I, Parkkinen L, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Morris J, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2008) Assessment of α-synuclein pathology: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol 67:125–143
Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Parkkinen L, Patsouris E, Roggendorf W, Rozemuller A, Stadelmann-Nessler C, Streichenberger N, Thal DR, Kretzschmar H (2009) Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol 117:635–652
Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ (2006) Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281:29739–29752
Attems J, Jellinger KA (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol Appl Neurobiol 34:466–467
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64:1404–1410
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Braak H, Del Tredici K (2008) Nervous system pathology in sporadic Parkinson disease. Neurology 70:1916–1925
Bruck A, Aalto S, Rauhala E, Bergman J, Marttila R, Rinne JO (2009) A follow-up study on 6-[18F]fluoro-l-dopa uptake in early Parkinson’s disease shows nonlinear progression in the putamen. Mov Disord 24:1009–1015
Brundin P, Li JY, Holton JL, Lindvall O, Revesz T (2008) Research in motion: the enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci 9:741–745
Burke RE, Dauer WT, Vonsattel JPG (2008) The Braak staging scheme for Parkinson’s disease: a reappraisal. Ann Neurol 64:485–491
Burns SA, Moran CR, Pingerelli PL, Ozols VV, Burns RS (2008) Redefining the Lewy body: evidence of distinct protein composition and nonrandom assembly process (abstr). Mov Disord 23(Suppl 1):S22
Calabresi P, Mercuri NB, Di Filippo M (2009) Synaptic plasticity, dopamine and Parkinson’s disease: one step ahead. Brain 132:285–287
Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA (2008) Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol 67:523–531
Colosimo C, Hughes AJ, Kilford L, Lees AJ (2003) Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:852–856
Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, Boston H, Saftig P, Woulfe J, Feany MB, Myllykangas L, Schlossmacher MG, Tyynela J (2009) Cathepsin D expression level affects α-synuclein processing, aggregation, and toxicity in vivo. Mol Brain 2:5
Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN (1992) Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol 83:525–529
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080
Dickson DW, Fujishiro H, Delledonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115:437–444
Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ (2002) Concurrence of α-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol 104:7–11
Fearnley JM, Lees AJ (1994) Pathology of Parkinson’s disease. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 545–554
Forno LS (1969) Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc 17:557–575
Fujimi K, Sasaki K, Noda K, Wakisaka Y, Tanizaki Y, Matsui Y, Sekita A, Iida M, Kiyohara Y, Kanba S, Iwaki T (2008) Clinicopathological outline of dementia with Lewy bodies applying the revised criteria: the Hisayama study. Brain Pathol 18:317–325
Fujishiro H, Ahn TB, Frigerio R, Uchikado H, Klos KJ, Josephs KA, DelleDonne A, Parisi JE, Ahlskog JE, Dickson DW (2008) Incidental Lewy bodies in various neurodegenerative disorders (abstr.). Mov Disord 23(Suppl.1):S30
Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW (2008) Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol 67:649–656
Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW (2008) Co-localization of tau and α-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol 116:269–275
Galpern WR, Lang AE (2006) Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol 59:449–458
Galvin JE, Lee VM, Trojanowski JQ (2001) Synucleinopathies: clinical and pathological implications. Arch Neurol 58:186–190
Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, Piette F, Hauw JJ, Duyckaerts C (2006) Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63:584–588
Greffard S, Verny M, Bonnet AM, Seilhean D, Hauw JJ, Duyckaerts C (2008) A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol Aging (online May 3, 2008)
Halliday G, Hely M, Reid W, Morris J (2008) The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol 115:409–415
Halliday GM, McRitchie DA, Cartwright H, Pamphlett R, Hely MA, Morris JGL (1996) Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J Clin Neurosci 3:52–60
Halliday GM, Del Tredici K, Braak H (2006) Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm Suppl 70:99–103
Hamilton RL (2000) Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 10:378–384
Harding AJ, Halliday GM (2001) Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol 102:355–363
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23:837–844
Ince PG, Clark B, Holton J, Revesz T, Wharton SB (2008) Disorders of movement and system degenerations. In: Love S, Louis DN, Ellison DW (eds) Greenfield’s neuropathology, 8th edn. Hodder Arnold, London, pp 889–1030
Ishiyama M, Yagishita S, Hasegawa K, Yokoyama T (2006) Ultrastructural study (tEM and sEM) of cortical Lewy bodies. Neuropathol 26:A58
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and α-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Jellinger KA (2004) Lewy body-related α-synucleinopathy in the aged human brain. J Neural Transm 111:1219–1235
Jellinger KA (2007) Lewy body disorders. In: Youdim MBH, Riederer P, Mandel SA, Battistin L, Lajtha A (eds) Degenerative diseases of the nervous system. Springer Science, New York, pp 267–343
Jellinger KA (2007) Morphological substrates of Parkinsonism with and without dementia. A retrospective clinico-pathological study. J Neural Transm Suppl 72:91–104
Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116:1–16
Jellinger KA (2009) A critical evaluation of current staging of α-synuclein pathology in Lewy body disorder. Biochim Biophys Acta 1792:730–740
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2008) Controversies over the staging of α-synuclein pathology in Parkinson’s disease. Acta Neuropathol 116:125–128
Kalaitzakis ME, Roncaroli F, Pearce RK, Gentleman S (2008) Non-stereotypical distribution of α-synuclein in the spinal cord and brain of a patient with dementia with Lewy bodies (DLB) (abstr.). Mov Disord 23(Suppl.1):S247
Kanazawa T, Uchihara TAT, Nakamura A, Orimo S, Mizusawa H (2008) Three-layered structure shared between Lewy bodies and Lewy neurites—three-dimensional reconstruction of triple-labeled sections. Brain Pathol 18:415–422
Katsuse O, Iseki E, Marui W, Kosaka K (2003) Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies. J Neurol Sci 211:29–35
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14:504–506
Kovacs GG, Milenkovic IJ, Preusser M, Budka H (2008) Nigral burden of α-synuclein correlates with striatal dopamine deficit. Mov Disord 23:1608–1612
Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2003) Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106:83–88
Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of Lewy bodies: dissimilar incorporation of α-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253
Lee HG, Zhu X, Takeda A, Perry G, Smith MA (2006) Emerging evidence for the neuroprotective role of α-synuclein. Exp Neurol 200:1–7
Lee VM, Giasson BI, Trojanowski JQ (2004) More than just two peas in a pod: common amyloidogenic properties of tau and α-synuclein in neurodegenerative diseases. Trends Neurosci 27:129–134
Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J (2007) Proteomic identification of novel proteins in cortical Lewy bodies. Brain Pathol 17:139–145
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ (2008) Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 18:220–224
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14:501–503
Licker V, Dayon L, Turck N, Côte M, Rodrigo N, Hochstrasser DF, Sanchez JC, Burkhard PR (2009) Proteomic analysis of the substantia nigra in patients with Parkinson’s disease (abstr.). Mov Disord 24(suppl 1):S39
Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D Jr, Price DL (2007) The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol 66:251–257
MacInnes N, Iravani MM, Perry E, Piggott M, Perry R, Jenner P, Ballard C (2008) Proteasomal abnormalities in cortical Lewy body disease and the impact of proteasomal inhibition within cortical and cholinergic systems. J Neural Transm 115:869–878
Markesbery WR, Jicha GA, Liu H, Schmitt FA (2009) Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 68:816–822
Marras C, Lang A (2008) Invited article: changing concepts in Parkinson disease: moving beyond the decade of the brain. Neurology 70:1996–2003
Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M (2000) α-Synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol 100:285–290
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
Meredith GE, Sonsalla PK, Chesselet MF (2008) Animal models of Parkinson’s disease progression. Acta Neuropathol 115:385–398
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Müller CM, de Vos RA, Maurage CA, Thal DR, Tolnay M, Braak H (2005) Staging of sporadic Parkinson disease-related α-synuclein pathology: inter- and intra-rater reliability. J Neuropathol Exp Neurol 64:623–628
Muntane G, Dalfo E, Martinez A, Ferrer I (2008) Phosphorylation of tau and α-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related α-synucleinopathies. Neuroscience 152:913–923
Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H (2008) Relationship of phosphorylated α-synuclein and tau accumulation to Aβ deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol 210:409–420
Outeiro TF, McLean PJ, Hyman BT (2007) Protein aggregation disorders. In: Gilman S (ed) Neurobiology of disease. Elsevier, Academic Press, Amsterdam, pp 111–123
Paleologou KE, Kragh CL, Mann DM, Salem SA, Al-Shami R, Allsop D, Hassan AH, Jensen PH, El-Agnaf OM (2009) Detection of elevated levels of soluble α-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain 132:1093–1101
Parkkinen L, Soininen H, Alafuzoff I (2003) Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 62:363–367
Parkkinen L, Pirttila T, Tervahauta M, Alafuzoff I (2005) Widespread and abundant α-synuclein pathology in a neurologically unimpaired subject. Neuropathology 25:304–314
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407
Peuralinna T, Oinas M, Polvikoski T, Paetau A, Sulkava R, Niinisto L, Kalimo H, Hernandez D, Hardy J, Singleton A, Tienari PJ, Myllykangas L (2008) Neurofibrillary tau pathology modulated by genetic variation of α-synuclein. Ann Neurol 64:348–352
Power JH, Blumbergs PC (2009) Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol 117:63–73
Probst A, Bloch A, Tolnay M (2008) New insights into the pathology of Parkinson’s disease: does the peripheral autonomic system become central? Eur J Neurol 15(Suppl):1–4
Riederer P, Gerlach M, Muller T, Reichmann H (2007) Relating mode of action to clinical practice: dopaminergic agents in Parkinson’s disease. Parkinsonism Relat Disord 13:466–479
Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S (2004) Lewy body-related α-synucleinopathy in aging. J Neuropathol Exp Neurol 63:742–749
Schlossmacher MG (2007) α-Synuclein and synucleinopathies. In: Growdon JH, Rossor MN (eds) The Dementias 2. Blue books of neurology. Butterworth-Heinemann, Oxford, pp 184–213
Shehadeh L, Mitsi G, Adi N, Bishopric N, Papapetropoulos S (2009) Expression of Lewy body protein septin 4 in postmortem brain of Parkinson’s disease and control subjects. Mov Disord 24:204–210
Sossi V, de la Fuente-Fernandez R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ (2007) Dopamine transporter relation to dopamine turnover in Parkinson’s disease: a positron emission tomography study. Ann Neurol 62:468–474
Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F, Wakabayashi K, Itoyama Y (2008) Serine 129 phosphorylation of α-synuclein induces unfolded protein response-mediated cell death. J Biol Chem 283:23179–23188
Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM (2004) Aggresomes formed by α-synuclein and synphilin-1 are cytoprotective. J Biol Chem 279:4625–4631
Tofaris GK, Spillantini MG (2005) α-Synuclein dysfunction in Lewy body diseases. Mov Disord 20(Suppl 12):S37–S44
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of α-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
Uversky VN (2007) Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem 103:17–37
Volles MJ, Lansbury PT Jr (2003) Zeroing in on the pathogenic form of α-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42:7871–7878
Wakabayashi K, Mori F, Takahashi H (2006) Progression patterns of neuronal loss and Lewy body pathology in the substantia nigra in Parkinson’s disease. Parkinsonism Relat Disord 12(Suppl):S92–S98
Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T (2003) Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 106:374–382
Waxman EA, Duda JE, Giasson BI (2008) Characterization of antibodies that selectively detect α-synuclein in pathological inclusions. Acta Neuropathol 116:37–46
Weisman D, Cho M, Taylor C, Adame A, Thal LJ, Hansen LA (2007) In dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology 69:356–359
Wolters E, Braak H (2006) Parkinson’s disease: premotor clinico-pathological correlations. J Neural Transm Suppl 70:309–319
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG (2008) Patterns and stages of α-synucleinopathy: relevance in a population-based cohort. Neurology 70:1042–1048
Conflict of interest statement
The author has no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
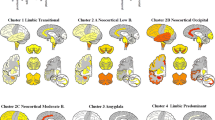
After submission of this manuscript, a new unified staging system for LB disorders, based on examination of a standard set of 10 brain regions in a large number of autopsy cases suggested four stages of LB pathology: (I) olfactory bulb only; (IIa) brainstem predominant; (IIb) limbic predominant; (III) brainstem and limbic; (IV) neocortical. Progression of these stages was correlated with nigrostriatal degeneration, cognitive impairment and motor dysfunction. The proposed staging system was suggested to improve classification of LB disorders [6]. The results of staging/typing of LB-related αSyn pathology of the BrainNet Europe Consortium were published in the same fascicle of ANP [3].
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Formation and development of Lewy pathology: a critical update. J Neurol 256 (Suppl 3), 270–279 (2009). https://doi.org/10.1007/s00415-009-5243-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5243-y