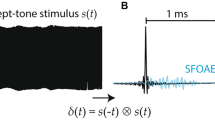
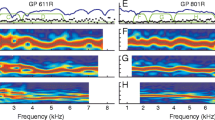
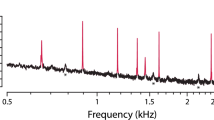
Abstract
Many non-mammalian ears lack physiological features considered integral to the generation of otoacoustic emissions in mammals, including basilar-membrane traveling waves and hair-cell somatic motility. To help elucidate the mechanisms of emission generation, this study systematically measured and compared evoked emissions in all four classes of tetrapod vertebrates using identical stimulus paradigms. Overall emission levels are largest in the lizard and frog species studied and smallest in the chicken. Emission levels in humans, the only examined species with somatic hair cell motility, were intermediate. Both geckos and frogs exhibit substantially higher levels of high-order intermodulation distortion. Stimulus frequency emission phase-gradient delays are longest in humans but are at least 1 ms in all species. Comparisons between stimulus-frequency emission and distortion-product emission phase gradients for low stimulus levels indicate that representatives from all classes except frog show evidence for two distinct generation mechanisms analogous to the reflection- and distortion-source (i.e., place- and wave-fixed) mechanisms evident in mammals. Despite morphological differences, the results suggest the role of a scaling-symmetric traveling wave in chicken emission generation, similar to that in mammals, and perhaps some analog in the gecko.








Similar content being viewed by others
Notes
By generation mechanism we mean the totality of processes that contribute to creating an OAE. Mechanisms encompass the forward propagation path for the evoking stimuli, the production of reverse traveling waves within the inner ear, and the reverse propagation path to the microphone. For example, a mechanism in one species might comprise a mechanical lever (middle ear), a delay line (BM traveling wave), and a group of nonlinear oscillators (hair cells).
Frost et al. (2006) have called for a restructuring of the amphibian taxonomy. In their proposed reclassification, leopard frogs are no longer designated as Rana pipiens pipiens.
For all species where repeated measurements were made in an individual ear during separate experimental sessions, as many as four separate observations may have been made. For data shown in the results section where repeated data from an individual ear is excluded (to avoid bias), the data set that is included was chosen at random. All subjects/animals were healthy to the best of our knowledge during the interval between sessions.
Given the direct coupling between the oral cavity and the middle ear space in non-mammals, OAE measurements could presumably be affected by whether the animal’s mouth is open or closed. Provided the calibration was rerun after any changes in the animal’s posture, we did not see any evidence over the course of the present study that indicated that emissions are sensitive to whether the animal’s mouth is opened or closed. For the sake of consistency, we attempted to make sure the animal’s mouth was open for all OAE recordings, though there was some variability in this regard with the chickens.
As addressed briefly in the “Discussion”, a limited number of experiments were made over the course of the present study where the body temperature of the gecko was varied. The presence of frequency notches was clearly (and reversibly) temperature-dependent.
Similar values are obtained when half-octave bins are used.
Measurements of DPOAEs in several frogs using a fixed f 2/f 1 ratio of 1.22 and stimulus intensities (L 1 = L 2) ranging from 45 to 75 dB SPL provided no indication that the 2f 1−f 2 phase flattens out relative to that of 2f 2−f 1 or the SFOAE (although phase gradients for a given OAE did vary some with level). DPOAEs at primary levels below 65 dB SPL for ratios other than 1.22 were not measured in this study for the frog.
Based upon differences in the DPOAE physiological vulnerability in the frog ear, van Dijk et al. (2003) indicated a distinction between low and high-level emissions. Their distinction is different however than the one we make here: we are primarily concerned with the linearity of the OAE response. How the distinctions between low and high-level emission generation mechanisms made here and that made by van Dijk et al. are related is not presently clear.
Within the context of cochlear traveling waves, local scaling implies that the number of wavelengths (i.e., the total amount of phase accumulation) between the stapes and the peak of the traveling wave varies only slowly with frequency.
A mechanical delay stemming from a traveling wave in the TM of the AP could explain the long ANF time delays (in addition to the eOAE phase-gradient delays) observed in the frog, which do not appear to be associated with mechanical tuning. The frog has the smallest Q 10 values of all the non-human species tested in the present study.
Shera and Guinan (2003) provide a discussion on the connection between Q ERB (equivalent rectangular bandwidth) and Q 10 (in their footnote 6).
The microphonic is a gross electrical responses measured at the round window with an electrode. Wever’s (1978) choice of a 1 μV microphonic threshold criterion appears to correlate well with ANF-derived thresholds, at least at lower frequencies. At higher frequencies, the bi-directional orientation of the hair bundles produces BM cancellation, creating the impression of a higher threshold (Eatock et al. 1981).
Abbreviations
- τ OAE :
-
emission phase-gradient delay
- ANF:
-
auditory nerve fiber
- AP:
-
amphibian papilla
- BM:
-
basilar membrane
- BP:
-
basilar papilla
- DPOAE:
-
distortion-product otoacoustic emission
- eOAE:
-
evoked otoacoustic emission
- Q :
-
quality factor
- SFOAE:
-
stimulus-frequency otoacoustic emission
- SOAE:
-
spontaneous otoacoustic emission
- TM:
-
tectorial membrane
References
Aranyosi AJ, Freeman DM (2004) Sound-induced motions of individual cochlear hair bundles. Biophys J 87(5):3536–3546
Boyev KP, Liberman MC, Brown MC (2002) Effects of anesthesia on efferent-mediated adaptation of the DPOAE. J Assoc Res Otolaryngol 3(3):362–373
Brass D, Kemp DT (1993) Suppression of stimulus frequency otoacoustic emissions. J Acoust Soc Am 93(2):920–939
Brown AM, Kemp DT (1983) An integrated view of cochlear mechanical nonlinearities observable from the ear canal. In: deBoer E, Viergever MA (eds) Mechanics of hearing. Martinus Nijhoff, The Hague, pp 75–82
Brownell WE, Bader CR, Bertrand D, Ribaupierre YD (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science 227:194–196
Chen L, Salvi R, Shero M (1994) Cochlear frequency-place map in adult chickens: intracellular biocytin labeling. Hear Res 81(1):130–136
Coro F, Kössl M (1998) Distortion-product otoacoustic emissions from the tympanic organ in two noctuoid moths. J Comp Physiol A 183:525–531
Coro F, Kössl M (2001) Components of the 2f1–f2 distortion-product otoacoustic emission in a moth. Hear Res 162:126–133
Cotanche DA (1987) Regeneration of the tectorial membrane in the chick cochlea following severe acoustic trauma. Hear Res 30:197–206
Dallos P, Popper AN, Fay RR (eds) (1996) The cochlea. Springer, New York
Eatock RA, Manley GA, Pawson L (1981) Auditory nerve fiber activity in the gecko. I. Implications for cochlear processing. J Comp Physiol A 142:203–218
Ferber-Viart C, Savourey G, Garcia C, Duclaux R, Bittel J, Collet L (1995) Influence of hyperthermia on cochlear micromechanical properties in humans. Hear Res 91(1–2):202–207
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, de S RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297:1–370
Ghaffari R, Aranyosi AJ, Freeman DM (2007) Longitudinally propagating traveling waves of the mammalian tectorial membrane. Proc Natl Acad Sci 104(42):16510–16515
Glasberg BR, Moore BC (2000) Frequency selectivity as a function of level and frequency measured with uniformly exciting notched noise. J Acoust Soc Am 108(5):2318–2328
Goodman SS, Withnell RH, Shera CA (2003) The origin of SFOAE microstructure in the guinea pig. Hear Res 183:7–17
Gummer AW, Smolders JWT, Klinke R (1987) Basilar membrance motion in the pigeon measured with the Mössbauer technique. Hear Res 29:63–92
He DZ, Beisel KW, Chen L, Ding DL, Jia S, Fritzsch B, Salvi R (2003) Chick hair cells do not exhibit voltage-dependent somatic motility. J Physiol 546(2):511–520
Hellmann B, Fritzsch B (1996) Neuroanatomical and histochemical evidence for the presence of common lateral line and inner ear efferents and of efferents to the basilar papilla in a frog, Xenopus laevis. Brain Behav Evol 47:185–194
Hillery CM, Narins PM (1984) Neurophysiological evidence for a traveling wave in the amphibian inner ear. Science 225:1037–1039
Ipakchi R, Kyin T, Saunders JC (2005) Loss and recovery of sound-evoked otoacoustic emissions in young chicks following acoustic trauma. Audiol Neurootol 10(4):209–219
Kemp DT (1986) Otoacoustic emissions, travelling waves and cochlear mechanisms. Hear Res 22:95–104
Kennedy HJ, Evans MG, Crawford AC, Fettiplace R (2006) Depolarization of cochlear outer hair cells evokes active hair bundle motion by two mechanisms. J Neurosci 26(10):2757–2766
Kettembeil S, Manley GA, Siegl E (1995) Distortion-product otoacoustic emissions and their anesthesia sensitivity in the European starling and the chicken. Hear Res 86:47–62
Knight RD, Kemp DT (2000) Indications of different distortion product otoacoustic emission mechanisms from a detailed f 1,f 2 area study. J Acoust Soc Am 107(1):457–473
Köppl C (1995) Otoacoustic emissions as an indicator for active cochlear mechanics: a primitive property of vertebrate auditory organs. In: Manley GA et al (eds) Advances in hearing research. World Scientific, Singapore, pp 207–218
Köppl C, Forge A, Manley GA (2004) Low density of membrane particles in auditory hair cells of lizards and birds suggests an absence of somatic motility. J Comp Neurol 479(2):149–155
Kössl M, Boyan GS (1998) Acoustic distortion products from the ear of a grasshopper. J Acoust Soc Am 104(1):326–335
Lewis ER, Leverenz EL (1983) Morphological basis for tonotopy in the anuran amphibian papilla. Scan Electron Microsc 1:189–200
Lewis ER, Leverenz EL, Koyama H (1982) The tonotopic organization of the bullfrog amphibian papilla, an auditory organ lacking a basilar membrane. J Comp Physiol A 145:437–445
Liberman MC, Zuo J, Guinan JJ (2004) Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea?. J Acoust Soc Am 116(3):1649–1655
Lichtenhan JT, Chertoff ME, Smittkamp SE, Durham D, Girod DA (2005) Predicting severity of cochlear hair cell damage in adult chickens using DPOAE input–output functions. Hear Res 201(1–2):109–120
Long GR, Talmadge CL (2007) DPOAE fine structure changes at higher stimulus levels—evidence for a nonlinear reflection component. In: Nuttall AL et al (eds) Auditory mechanisms: processes and models. World Scientific, Singapore, pp 287–293
Manley GA (1990) Peripheral hearing mechanisms in reptiles and birds. Springer, Berlin
Manley GA, Köppl C (1994) Spontaneous otoacoustic emissions in the bobtail lizard. III: temperature effects. Hear Res 72(1–2):171–180
Manley GA, Brix J, Kaiser A (1987) Developmental stability of the tonotopic organization of the chick’s basilar papilla. Science 237:655–656
Manley GA, Yates GK, Köppl C (1988) Auditory peripheral tuning: evidence for a simple resonance phenomenon in the lizard Tiliqua. Hear Res 33:181–190
Manley GA, Köppl C, Johnstone BM (1993) Distortion-product otoacoustic emissions in the bobtail lizard. I: general characteristics. J Acoust Soc Am 93(5):2820–2833
Manley GA, Gallo L, Köppl C (1996) Spontaneous otoacoustic emissions in two gecko species, Gekko gecko and Eublepharis macularius. J Acoust Soc Am 99(3):1588–1603
Manley GA, Köppl C, Sneary M (1999) Reversed tonotopic map of the basilar papilla in Gekko gecko. Hear Res 131(1–2):107–116
Martin GK, Stagner BB, Jassir D, Telischi FF, Lonsbury-Martin BL (1999) Suppression and enhancement of distortion-product otoacoustic emissions by interference tones above f(2). I. Basic findings in rabbits. Hear Res 136(1–2):105–123
Meenderink SW (2005) Distortion product otoacoustic emissions from the anuran ear. Ph.D. Thesis, University of Maastricht
Meenderink SW, Narins PM (2006) Stimulus frequency otoacoustic emissions in the Northern leopard frog, Rana pipiens pipiens: implications for inner ear mechanics. Hear Res 220(1–2):67–75
Meenderink SW, van Dijk P (2004) Level dependence of distortion product otoacoustic emissions in the leopard frog, Rana pipiens pipiens. Hear Res 192:107–118
Meenderink SW, van Dijk P (2006) Temperature dependence of anuran distortion product otoacoustic emissions. J Assoc Res Otolaryngol 7(3):246–252
Meenderink SW, van Dijk P, Narins PM (2005) Detailed f 1, f 2 area study of distortion product otoacoustic emissions in the frog. J Assoc Res Otolaryngol 6:37–47
Neely ST, Kim DO (1983) An active cochlear model showing sharp tuning and high selectivity. Hear Res 9(2):123–130
Peake PT, Ling A Jr (1980) Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J Acoust Soc Am 67(5):1736–1745
Ronken DA (1991) Spike discharge properties that are related to the characteristic frequency of single units in the frog auditory nerve. J Acoust Soc Am 90(5):2428–2440
Rosowski JJ, Peake WT, White JR (1984) Cochlear nonlinearities inferred from two-tone distortion products in the ear canal of the alligator lizard. Hear Res 13(2):141–158
Salvi RJ, Saunders SS, Powers NL, Boettcher FA (1992) Discharge patterns of the cochlear ganglion neurons in the chicken. J Comp Physiol A 170: 227–241
Sams-Dodd F, Capranica RR (1994) Representation of acoustic signals in the eighth nerve of the Tokay gecko: I. Pure tones. Hear Res 76:16–30
Saunders SS, Salvi RJ (1993) Psychoacoustics of normal adult chickens: thresholds and temporal integration. J Acoust Soc Am 94(1):83–90
Schairer KS, Ellison JC, Fitzpatrick D, Keefe DH (2006) Use of stimulus-frequency otoacoustic emission latency and level to investigate cochlear mechanics in human ears. J Acoust Soc Am 120(2):901–914
Shera CA (2003) Wave interference in the generation of reflection- and distortion-source emissions. In: Gummer AW et al (eds) Biophysics of the cochlea: from molecules to models. World Scientific, Singapore, pp 439–453
Shera CA, Guinan JJ Jr (1999) Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 105(2):782–798
Shera CA, Guinan JJ Jr (2003) Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am 113(5):2762–2772
Shera CA, Guinan JJ Jr (2007) Cochlear traveling-wave amplification, suppression, and beamforming probed using noninvasive calibration of intracochlear distortion sources. J Acoust Soc Am 121(2):1003–1016
Shera CA, Guinan JJ Jr, Oxenham AJ (2002) Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci 99(5):3318–3323
Shofner WP, Feng AS (1983) A quantitative light microscopic study of the bullfrog amphibian papilla tectorium: correlation with tonotopic organization. Hear Res 11:103–116
Stewart CE, Hudspeth AJ (2000) Effects of salicylates and aminoglycosides on spontaneous otoacoustic emissions in the Tokay gecko. Proc Natl Acad Sci 97(1):454–459
Talmadge CL, Tubis A, Long GR, Piskorski P (1998) Modeling otoacoustic emission and hearing threshold fine structures. J Acoust Soc Am 104(3): 1517–1543
Talmadge CL, Tubis A, Long GR, Tong C (2000) Modeling the combined effects of basilar membrane nonlinearity and roughness on stimulus frequency otoacoustic emission fine structure. J Acoust Soc Am 108(6): 2911–2932
Tanaka K, Smith CA (1975) Structure of the avian tectorial membrane. Ann Otol Rhinol Laryngol 84(3 pt. 1):287–296
Tanaka K, Smith CA (1978) Structure of the chicken’s inner ear: SEM and TEM study. Am J Anat 153(2):251–271
Taschenberger G, Manley GA (1997) Spontaneous otoacoustic emissions in the barn owl. Hear Res 110:61–76
Tilney LG, Saunders JC (1983) Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol 96(3):807–821
Ulehlova L, Voldrich L, Janisch R (1987) Correlative study of sensory cell density and cochlear length in humans. Hear Res 28:149–151
van der Heijden M, Joris PX (2006) Panoramic measurements of the apex of the cochlea. J Neurosci 26(44):11462–11473
van Dijk P, Narins PM, Wang J (1996) Spontaneous otoacoustic emissions in seven frog species. Hear Res 101:102–112
van Dijk P, Mason MJ, Narins PM (2002) Distortion product otoacoustic emissions in frogs: correlation with middle and inner ear properties. Hear Res 173:100–108
van Dijk P, Narins PM, Mason MJ (2003) Physiological vulnerability of distortion product otoacoustic emissions from the amphibian ear. J Acoust Soc Am 114(4):2044–2048
Veuillet E, Gartner M, Champsaur G, Neidecker J, Collet L (1997) Effects of hypothermia on cochlear micromechanical properties in humans. J Neurol Sci 145(1):69–76
von Bekesy G (1960) Experiments in hearing. McGraw-Hill, New York
Werner YL (1976) Optimal temperatures for inner-ear performance in gekkonoid lizards. J Exp Zool 195(3):319–352
Wever EG (1973) The ear and hearing in the frog, Rana pipiens. J Morphol 141:461–478
Wever EG (1978) The reptile ear. Princeton University Press, Englewood Cliffs
Wever EG (1985) The amphibian ear. Princeton University Press, Englewood Cliffs
Whitehead ML, Stagner BB, Martin GK, Lonsbury-Marin BL (1996) Visualization of the onset of distortion product otoacoustic emissions, and measurement of their latency. J Acoust Soc Am 100(3):1663–1679
Zweig G, Shera CA (1995) The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98(4):2018–2047
Acknowledgments
The authors acknowledge valuable discussions and comments from AJ Aranyosi, Paul Fahey, John Guinan, and John Rosowski. Comments from two anonymous reviewers are also gratefully acknowledged. This work was supported by NIH grants T32 DC00038 (CB), R01 DC000238 (DMF), R01 DC000710 (JCS), and R01 DC003687 (CAS). The experiments involving animals comply with the “Principles of animal care”, publication No. 86-23, revised 1985 of the National Institute of Health and with the current laws of the United States. Experiments involving humans were carried out with the approval of the Massachusetts Institute of Technology Committee On the Use of Humans as Experimental Subjects and the Human Studies Committee at the Massachusetts Eye and Ear Infirmary.
Author information
Authors and Affiliations
Corresponding author
Appendix: Anatomical overview
Appendix: Anatomical overview
This section provides background on the anatomy and physiology of peripheral auditory structures potentially relevant to OAE generation.
All the species examined here have a tympanic membrane enclosing the middle ear, although the presence of a tympanic membrane is unnecessary for the detection of DPOAEs (van Dijk et al. 2002). In contrast to mammals, the middle ear in the non-mammalian species examined here consists of a single bone, the columella, that couples the tympanic membrane directly to the stapedial footplate. The middle ears of both mammals and non-mammals play similar functional roles, providing both forward (stimulus going in) and reverse (OAE coming out) transmission to and from the inner ear.
The greatest amount of diversity across the examined species lies in the inner-ear anatomy (Fig. 9). Analogous to the organ of Corti in the mammalian cochlea, bird and lizard hair cells are situated in a structure called the basilar papilla (BP). The frog has two morphologically distinct auditory papilla in the inner ear. Specifics of the inner ear structures of each species are summarized below. The main qualitative differences in inner ear anatomy and physiology are summarized in Table 2. For brevity, we provide only brief descriptions of human cochlear anatomy and hearing perception (see Dallos et al. 1996). The term “cochlea” is reserved here for the auditory organ of the mammalian inner ear.
Comparative schematic of inner-ear anatomy. Two perspectives are provided for each group: a cross-sectional view (left) and a top-down view of a section of the sensory epithelium (right). Except for the frog, the arrows in the top-down view represent an individual hair cell, the direction indicating the bundle’s polarization (pointing from shortest to tallest row). For the frog, the entire longitudinal length of only the amphibian papilla (AP) is shown and arrows indicate gross trends of the hair cells (the finely dashed bounding box corresponds to where the cross-section would lay and the coarsely dashed line represents where the sensing membrane extends down from the roof of the recess). Cells known to exhibit cell body somatic motility are indicated by a star on their tail. White regions are fluid-filled, grey regions correspond to overlying tectorium (with grey lines indicating fibrillar structure), grey striped area represents bone and stippled areas are non-stereociliary cellular regions (e.g., supporting cells). Distinction between scala vestibuli and scala media is omitted. Legend is as follows: AP amphibian papilla, AR amphibian recess, BM basilar membrane, BP basilar papilla, FN fundus, LL limbic lip, SA sallet, SC sallet chain, SE sensing membrane, SM scala media, ST scala tympani, TC tunnel of Corti, TM tectorial membrane
Humans
The human cochlea (i.e., BM length) is typically 33–35 mm in length (coiled over two and a half turns) and contains around 15,000 total hair cells (Ulehlova et al. 1987). Typical thresholds in a healthy human ear are relatively flat between 0.5 and 7 kHz, being in the range of −5 to 15 dB SPL. Peak sensitivity occurs in the frequency range of 3–4 kHz. Psychophysical human Q ERB values typically range from 7 to 10 around 1 kHz to 9–17 around 4 kHz (e.g., Glasberg and Moore 2000; Shera et al. 2002) Footnote 13.
Chickens
Chickens have a short BM (∼3–4 mm) that curves gently over its length. The BM width and thickness change along its length (as well as hair-cell bundle properties such as height and number of stereocilia), correlating to the tonotopic gradient observed from ANF responses (Manley et al. 1987; Chen et al. 1994). Evidence from avian species suggests that a longitudinally traveling wave is present along the BM (von Bekesy 1960; Gummer et al. 1987). There are ≈5,000 hair cells situated in a hexagonal fashion (Tilney and Saunders 1983). In general, two distinct types of hair cells have been characterized: short hair cells sitting directly atop the BM (receiving the bulk of the efferent innervation) and tall hair cells (with the bulk of the afferent innervation) (Tanaka and Smith 1978). Chick hair cells do not exhibit somatic motility (He et al 2003; Köppl et al. 2004). The overlying TM is relatively quite thick, with dense radial and longitudinal fibers apparent under a light microscope. Cavities that are present in the TM over each hair cell extend back towards the homogene cells (at the neural edge), making the TM appear porous through a given cross-section (Cotanche 1987). For all hair cells in the papilla, the tallest row of the stereociliary bundle is tightly coupled to the TM, which is also attached to the papillar surface via fibrillar connections coupling to the microvilli of the supporting cells (Tanaka and Smith 1975).
Based upon ANF recordings from previous studies, P21 (number of days post-hatching) chicks have a flat mean threshold of ≈20 dB SPL from 0.2 to 3 kHz, increasing sharply at higher frequencies (Manley 1990). Psychoacoustic studies in adult chickens correlate well to these measurements (Saunders and Salvi 1993). Q 10 values from the single units are typically around 2–5 (though some units exhibit significantly higher Q values), and increase with characteristic frequency (Salvi et al. 1992). While DPOAEs have been measured in the chicken (Kettembeil et al. 1995; Ipakchi et al. 2005; Lichtenhan et al. 2005), the authors know of no published reports of spontaneous emissions (SOAEs) or SFOAEs in Gallus gallus domesticus.
Geckos
Two species of gecko were examined in this study: Leopard geckos (E. macularius) and Tokay geckos (G. gecko). Both have similar peripheral auditory anatomy (Wever 1978), including a short (1.2–1.8 mm) and straight BM. Both the width and thickness of the BM and BP vary considerably over the longitudinal length. The BP contains ≈1,000–2,000 hair cells (Wever 1978). The BM in G. gecko is slightly longer and supports ≈40% more hair cells. Hair-cell bundles are oriented both uni-directionally (all in the same direction) and bi-directionally (180° relative to one another). There is a unique tectorial topology along one region of the papilla that consists of sallets, discretized sections of TM loosely coupled to each other via a fine strand overlying their top surface called the sallet chain. The sallets couple a single row of bi-directionally oriented hair cells together as shown in Fig. 9 (Wever 1978). Evidence suggests the absence of both somatic hair-cell motility (Köppl et al. 2004) and traveling waves (Peake and Ling 1980; Manley et al. 1988, 1999) along the gecko BM. A thickened tissue called the fundus runs along the length of the BM underneath the BP. Both afferent and efferent innervations are present, though the latter appears exclusive to the uni-directional segment of the papilla (Manley 1990).
Previous studies have looked at microphonic responses in both species (Wever 1978) and ANF responses in G. gecko (Eatock et al. 1981; Sams-Dodd and Capranica 1994; Manley et al. 1999), giving an indication of the thresholds and sharpness of tuning Footnote 14. Based upon microphonic and ANF data, the G. gecko ear has a threshold of ≈10–15 dB SPL in its most sensitive region of 0.5–0.8 kHz. E. macularius appears to be a further 10–15 dB more sensitive than G. gecko based upon microphonic comparisons, suggesting thresholds at or below 0 dB SPL. Derived Q 10 values from the ANF studies for G. gecko were ∼2–4, increasing with characteristic frequency. Spontaneous emissions have been reported in both E. macularius and G. gecko (Manley et al. 1996; Stewart and Hudspeth 2000), but the present authors are unaware of any previous reports of evoked emissions in either gecko species.
Frogs
Frogs have two papillae that are sensitive to sound, the amphibian papilla (AP) and the basilar papilla (BP) (Wever 1985). In contrast to chicks and geckos, both papillae in frogs lack a flexible BM altogether, and the hair cells sit atop relatively rigid tissue (Wever 1973). Unlike those of the human and chicken, the hair cells in the papillae do not exhibit any obvious morphological distinctions (such as short vs. tall hair cells), but do exhibit a degree of bi-directionality (similar to that seen in the gecko). Shaped roughly like a horseshoe and ∼0.5–0.6 mm long (Wever 1973), the AP is tonotopically organized (Lewis et al. 1982) and is sensitive to frequencies below ∼1.2 kHz. Containing ≈800 hair cells, the AP has a thick TM punctuated by many small holes (Wever 1973). The hair cells couple tightly to the TM. A tectorial curtain (or sensing membrane extends from the bony roof of the AP recess down to the central portion of the TM. There does not appear to be a smooth gradation in either bundle or TM properties along the length of the AP (Shofner and Feng 1983; Lewis and Leverenz 1983). The BP, sensitive to higher frequencies (above ≈1.3 kHz) is smaller, containing only about 70 hair cells. The BP is thought to act as a singly tuned resonator (Ronken 1991). Unlike the AP, the BP does not appear to receive any efferent innervation in ranid frogs (Ronken 1991), though efferent innervation to the BP has been found in other amphibian species (Hellmann and Fritzsch 1996). Similar to lizards, a great deal of diversity is seen in the inner ear anatomy across different species of frogs.
Microphonic measurements in other frog species of the same family examined here indicate airborne thresholds near ∼20–40 dB SPL, being smallest in the range 0.2–0.6 kHz (Wever 1985). ANF responses in L. pipiens revealed higher mean thresholds, typically 50 dB SPL around 0.5–1 kHz and increasing at both lower and higher frequencies (Ronken 1991). Q 10 values range between 1 and 2, increasing at frequencies below 0.5 kHz and above 2 kHz (Ronken 1991). The existence of SOAEs has been reported for L. pipiens (van Dijk et al. 1996) while both DPOAEs (Meenderink et al. 2005) and SFOAEs (Meenderink and Narins 2006) have also been reported.
Rights and permissions
About this article
Cite this article
Bergevin, C., Freeman, D.M., Saunders, J.C. et al. Otoacoustic emissions in humans, birds, lizards, and frogs: evidence for multiple generation mechanisms. J Comp Physiol A 194, 665–683 (2008). https://doi.org/10.1007/s00359-008-0338-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-008-0338-y