Abstract
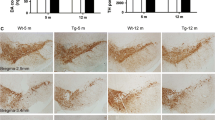
Proteinaceous inclusions, called Lewy bodies, are used as a pathological hallmark for Parkinson’s disease (PD). Lewy bodies contain insoluble α-synuclein (aSyn) and many other ubiquitinated proteins, suggesting a role for protein degradation system failure in the PD pathogenesis. Indeed, proteasomal dysfunction has been linked to PD but commonly used in vivo toxin models, such as 6-OHDA or MPTP, do not have a significant effect on the proteasomal system or protein aggregation. Therefore, we wanted to study the characteristics of a proteasomal inhibitor, lactacystin, as a PD model on young and adult mice. To study this, we performed stereotactic microinjection of lactacystin above the substantia nigra pars compacta in young (2 month old) and adult (12–14 month old) C57Bl/6 mice. Motor behavior was measured by locomotor activity and cylinder tests, and the markers of neuroinflammation, aSyn, and dopaminergic system were assessed by immunohistochemistry and HPLC. We found that lactacystin induced a Parkinson’s disease-like motor phenotype 5–7 days after injection in young and adult mice, and this was associated with widespread neuroinflammation based on glial cell markers, aSyn accumulation in substantia nigra, striatal dopamine decrease, and loss of dopaminergic cell bodies in the substantia nigra and terminals in the striatum. When comparing young and adult mice, adult mice were more sensitive for dopaminergic degeneration after lactacystin injection that further supports the use of adult mice instead of young when modeling neurodegeneration. Our data showed that lactacystin is useful in modeling various aspects of Parkinson’s disease, and taken together, our findings emphasize the role of a protein degradation deficit in Parkinson’s disease pathology, and support the use of proteasomal inhibitors as Parkinson’s disease models.






Similar content being viewed by others
References
Airavaara M, Mijatovic J, Vihavainen T et al (2006) In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse 59:321–329
Bentea E, Van der Perren A, Van Liefferinge J et al (2015) Nigral proteasome inhibition in mice leads to motor and non-motor deficits and increased expression of Ser129 phosphorylated alpha-synuclein. Front Behav Neurosci 9:68
Braak H, Sastre M, Del Tredici K (2007) Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241
Cuervo AM, Stefanis L, Fredenburg R et al (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295
Decressac M, Mattsson B, Lundblad M et al (2012) Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis 45:939–953
Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z et al (2011) Distinct roles in vivo for the ubiquitin–proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci 31:14508–14520
Ebrahimi-Fakhari D, McLean PJ, Unni VK (2012) Alpha-synuclein’s degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy 8:281–283
Elson JL, Yates A, Pienaar IS (2016) Pedunculopontine cell loss and protein aggregation direct microglia activation in parkinsonian rats. Brain Struct Funct 221:2319–2341
Fares M-B, Maco B, Oueslati A et al (2016) Induction of de novo α-synuclein fibrillization in a neuronal model for Parkinson’s disease. Proc Natl Acad Sci 113:E912–E921
Fenteany G, Schreiber SL (1998) Lactacystin, proteasome function, and cell fate. J Biol Chem 273:8545–8548
Fornai F, Lenzi P, Gesi M et al (2003) Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J Neurosci 23:8955–8966
Fornai F, Schlüter OM, Lenzi P et al (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin–proteasome system and alpha-synuclein. Proc Natl Acad Sci USA 102:3413–3418
Franklin K, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Galvan A, Wichmann T (2007) GABAergic circuits in the basal ganglia and movement disorders. Prog Brain Res 160:287–312
Gao HM, Kotzbauer PT, Uryu K et al (2008) Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28:7687–7698
Gerhard A, Banati RB, Goerres GB et al (2003) [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61:686–689
Goldbaum O, Riedel M, Stahnke T, Richter-Landsberg C (2009) The small heat shock protein HSP25 protects astrocytes against stress induced by proteasomal inhibition. Glia 57:1566–1577
Harrison IF, Crum WR, Vernon AC, Dexter DT (2015) Neurorestoration induced by the HDAC inhibitor sodium valproate in the lactacystin model of Parkinson’s is associated with histone acetylation and up-regulation of neurotrophic factors. Br J Pharmacol 172:4200–4215
Harrison IF, Anis HK, Dexter DT (2016) Associated degeneration of ventral tegmental area dopaminergic neurons in the rat nigrostriatal lactacystin model of parkinsonism and their neuroprotection by valproate. Neurosci Lett 614:16–23
Iannaccone S, Cerami C, Alessio M et al (2013) In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord 19:47–52
Imamura K, Hishikawa N, Sawada M et al (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106:518–526
Jin J, Meredith GE, Chen L et al (2005) Quantitative proteomic analysis of mitochondrial proteins: relevance to Lewy body formation and Parkinson’s disease. Brain Res Mol Brain Res 134:119–138
Kahle PJ, Neumann M, Ozmen L et al (2001) Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol 159:2215–2225
Kitada T, Asakawa S, Hattori N et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608
Konieczny J, Czarnecka A, Lenda T et al (2014a) Chronic l-DOPA treatment attenuates behavioral and biochemical deficits induced by unilateral lactacystin administration into the rat substantia nigra. Behav Brain Res 261:79–88
Konieczny J, Jantas D, Lenda T et al (2014b) Lack of neuroprotective effect of celastrol under conditions of proteasome inhibition by lactacystin in in vitro and in vivo studies: implications for Parkinson’s disease. Neurotox Res 26:255–273
Kumar A, Kopra J, Varendi K et al (2015) GDNF overexpression from the native locus reveals its role in the nigrostriatal dopaminergic system function. PLoS Genet 11:e1005710
Kwon SJ, Ahn TB, Yoon MY, Jeon BS (2008) BV-2 stimulation by lactacystin results in a strong inflammatory reaction and apoptotic neuronal death in SH-SY5Y cells. Brain Res 1205:116–121
Lee HJ, Suk JE, Patrick C et al (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285:9262–9272
Li C, Guo Y, Xie W et al (2010) Neuroprotection of pramipexole in UPS impairment induced animal model of Parkinson’s disease. Neurochem Res 35:1546–1556
Lorenc-Koci E, Lenda T, Antkiewicz-Michaluk L et al (2011) Different effects of intranigral and intrastriatal administration of the proteasome inhibitor lactacystin on typical neurochemical and histological markers of Parkinson’s disease in rats. Neurochem Int 58:839–849
Lou H, Montoya SE, Alerte TN et al (2010) Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem 285:17648–17661
Matsuoka Y, Vila M, Lincoln S et al (2001) Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis 8:535–539
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
McNaught KS, Jenner P (2001) Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci Lett 297:191–194
McNaught KS, Bjorklund LM, Belizaire R et al (2002) Proteasome inhibition causes nigral degeneration with inclusion bodies in rats. NeuroReport 13:1437–1441
McNaught KS, Belizaire R, Isacson O et al (2003) Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol 179:38–46
Meredith GE, Totterdell S, Petroske E et al (2002) Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res 956:156–165
Middeldorp J, Kamphuis W, Sluijs JA et al (2009) Intermediate filament transcription in astrocytes is repressed by proteasome inhibition. FASEB J 23:2710–2726
Mijatovic J, Airavaara M, Planken A et al (2007) Constitutive Ret activity in knock-in multiple endocrine neoplasia type B mice induces profound elevation of brain dopamine concentration via enhanced synthesis and increases the number of TH-positive cells in the substantia nigra. J Neurosci 27:4799–4809
Mijatovic J, Piltonen M, Alberton P et al (2011) Constitutive Ret signaling is protective for dopaminergic cell bodies but not for axonal terminals. Neurobiol Aging 32:1486–1494
Myöhänen TT, Hannula MJ, Van Elzen R et al (2012) A prolyl oligopeptidase inhibitor, KYP-2047, reduces alpha-synuclein protein levels and aggregates in cellular and animal models of Parkinson’s disease. Br J Pharmacol 166:1097–1113
Pan T, Kondo S, Le W, Jankovic J (2008a) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978
Pan T, Kondo S, Zhu W et al (2008b) Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis 32:16–25
Perez RG, Waymire JC, Lin E et al (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22:3090–3099
Pienaar IS, Harrison IF, Elson JL et al (2015) An animal model mimicking pedunculopontine nucleus cholinergic degeneration in Parkinson’s disease. Brain Struct Funct 220:479–500
Salin P, Manrique C, Forni C, Kerkerian-Le Goff L (2002) High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat. J Neurosci 22:5137–5148
Schallert T, Fleming SM, Leasure JL et al (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39:777–787
Shen YF, Tang Y, Zhang XJ et al (2013) Adaptive changes in autophagy after UPS impairment in Parkinson’s disease. Acta Pharmacol Sin 34:667–673
Snyder H, Mensah K, Theisler C et al (2003) Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem 278:11753–11759
Spillantini MG, Crowther RA, Jakes R et al (1998) alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473
St Martin JL, Klucken J, Outeiro TF et al (2007) Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J Neurochem 100:1449–1457
Stefanova N, Reindl M, Neumann M et al (2007) Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord 22:2196–2203
Tofaris GK, Razzaq A, Ghetti B et al (2003) Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem 278:44405–44411
Tofaris GK, Garcia Reitbock P, Humby T et al (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein (1–120): implications for Lewy body disorders. J Neurosci 26:3942–3950
Ulusoy A, Decressac M, Kirik D, Björklund A (2010) Viral vector-mediated overexpression of α-synuclein as a progressive model of Parkinson’s disease. Prog Brain Res 184:89–111
Vernon AC, Crum WR, Johansson SM, Modo M (2011) Evolution of extra-nigral damage predicts behavioural deficits in a rat proteasome inhibitor model of Parkinson’s disease. PLoS One 6:e17269
Wakabayashi K, Hayashi S, Yoshimoto M et al (2000) NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol 99:14–20
Wang Y, Zhang QJ, Liu J et al (2010) Changes in firing rate and pattern of GABAergic neurons in subregions of the substantia nigra pars reticulata in rat models of Parkinson’s disease. Brain Res 1324:54–63
Winner B, Jappelli R, Maji SK et al (2011) In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108:4194–4199
Wood SJ (1999) alpha-synuclein fibrillogenesis is nucleation-dependent. J Biol Chem 274:19509–19512
Acknowledgements
Authors would like to thank Susanna Norrbacka, Kati Rautio, and Liisa Lappalainen for excellent technical assistance. This work was supported by grants from the Academy of Finland [267788 and 2737991 (TTM) and 250275 (MA)], FinPharma Doctoral Programme (MS), Finnish Cultural Foundation (KA), the University of Helsinki grants (TTM), the Jane and Aatos Erkko Foundation (TTM.), the Sigrid Juselius Foundation (TTM), and the Emil Aaltonen Foundation (MS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest to declare.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and the use of experimental animals was approved by the Finnish National Board of Animal Experiments (ESAVI/198/04.10.07/2014).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Savolainen, M.H., Albert, K., Airavaara, M. et al. Nigral injection of a proteasomal inhibitor, lactacystin, induces widespread glial cell activation and shows various phenotypes of Parkinson’s disease in young and adult mouse. Exp Brain Res 235, 2189–2202 (2017). https://doi.org/10.1007/s00221-017-4962-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-4962-z