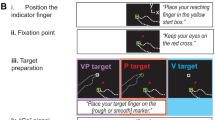
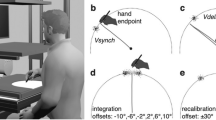
Abstract
While sensorimotor adaptation to prisms that displace the visual field takes minutes, adapting to an inversion of the visual field takes weeks. In spite of a long history of the study, the basis of this profound difference remains poorly understood. Here, we describe the computational issue that underpins this phenomenon and presents experiments designed to explore the mechanisms involved. We show that displacements can be mastered without altering the updated rule used to adjust the motor commands. In contrast, inversions flip the sign of crucial variables called sensitivity derivatives—variables that capture how changes in motor commands affect task error and therefore require an update of the feedback learning rule itself. Models of sensorimotor learning that assume internal estimates of these variables are known and fixed predicted that when the sign of a sensitivity derivative is flipped, adaptations should become increasingly counterproductive. In contrast, models that relearn these derivatives predict that performance should initially worsen, but then improve smoothly and remain stable once the estimate of the new sensitivity derivative has been corrected. Here, we evaluated these predictions by looking at human performance on a set of pointing tasks with vision perturbed by displacing and inverting prisms. Our experimental data corroborate the classic observation that subjects reduce their motor errors under inverted vision. Subjects’ accuracy initially worsened and then improved. However, improvement was jagged rather than smooth and performance remained unstable even after 8 days of continually inverted vision, suggesting that subjects improve via an unknown mechanism, perhaps a combination of cognitive and implicit strategies. These results offer a new perspective on classic work with inverted vision.





Similar content being viewed by others
References
Abdelghani MN, Tweed DB (2010) Learning course adjustments during arm movements with reversed sensitivity derivatives. BMC Neurosci 11:150
Abdelghani MN, Lillicrap TP, Tweed DB (2008) Sensitivity derivatives for flexible sensorimotor learning. Neural Comput 20(8):2085–2111
Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD (2010) Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22(9):1917–1930
Åström KJ, Wittenmark B (1995) Adaptive control, 2nd edn. Addison-Wesley, Reading
Block HJ, Bastian AJ (2012) Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia 50:1766–1775
Braun DA, Aertsen A, Wolpert DM, Mehring C (2009) Motor task variation induces structural learning. Curr Biol 19(4):352–357
Brinkman C, Porter R, Norman J (1983) Plasticity of motor behavior in monkeys with crossed forelimb nerves. Science 220(4595):438–440
Brooks V, Hilperath F, Brooks M, Ross HG, Freund HJ (1995) Learning “what” and “how” in a human motor task. Learn Mem 2(5):225–242
Callier FM, Desoer CA (1991) Linear system theory. Springer texts in electrical engineering. Springer-Verlag, New York
Cresswell AB, Macmillan AI, Hanna GB, Cuschieri A (1999) Methods for improving performance under reverse alignment conditions during endoscopic surgery. Surg Endosc 13(6):591–594
Cunningham HA (1989) Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol 15(3):493–506
Dean P, Porrill J, Stone JV (2002) Decorrelation control by the cerebellum achieves oculomotor plant compensation in simulated vestibulo-ocular reflex. Proc R Soc Lond B 269:1895–1904
Fernandez-Ruiz J, Diaz R, Moreno-Briseno P, Campos-Romo A, Ojeda R (2006) Rapid topographical plasticity of the visuomotor spatial transformation. J Neurosci 26(7):1986–1990
Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR (2011) Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res 219(1):8–14
Gritsenko V, Kalaska JF (2010) Rapid online correction is selectively suppressed during movement with a visuomotor transformation. J Neurophysiol 104:3084–3104
Harris CS (1965) Perceptual adaptation to inverted, reversed, and displaced vision. Psychol Rev 72(6):419–444
Illert M, Trauner M, Weller E, Wiedemann E (1986) Forearm muscles of man can reverse their function after tendon transfers: an electromyographic study. Neurosci Lett 67(2):129–134
Jordan MI, Rumelhart DE (1992) Forward models: supervised learning with a distal teacher. Cogn Sci 16(3):307–354
Kawato M, Gomi H (1992) The cerebellum and VOR/OKR learning models. Trends Neurosci 15(11):445–453
Kitazawa S, Kimura T, Uka T (1997) Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci 17(4):1481–1492
Kohler I (1963) The formation and transformation of the perceptual world. Psychological Issues, vol 3, No. 4, Monograph 12, International Universities Press, New York, pp 1–173
Kornheiser AS (1976) Adaptation to laterally displaced vision: a review. Psychol Bull 83(5):783–816
Linden DE, Kallenbach U, Heinecke A, Singer W, Goebel R (1999) The myth of upright vision. A psychophysical and functional imaging study of adaptation to inverting spectacles. Perception 28(4):469–481
Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT (1996) Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119(4):1199–1211
Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26(14):3642–3645
Mehta B, Schaal S (2002) Forward models in visuomotor control. J Neurophysiol 88(2):942–953
Moody J, Darken CJ (1989) Fast learning in networks of locally-tuned processing units. Neural Comput 1(2):281–294
Porrill J, Dean P, Stone JV (2004) Recurrent cerebellar architecture solves the motor-error problem. Proc Biol Sci R Soc Lond B 271(1541):789–796
Pouget A, Snyder LH (2000) Computational approaches to sensorimotor transformations. Nat Neurosci 3:1192–1198
Redding GM, Wallace B (1990) Effects on prism adaptation of duration and timing of visual feedback during pointing. J Mot Behav 22(2):209–224
Redding GM, Rossetti Y, Wallace B (2005) Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav R 29(3):431–444
Rock I (1966) The nature of perceptual adaptation. Basic Books, New York
Rock I (1973) Orientation and form. Academic Press, New York
Rolls ET, Deco G (2002) Computational neuroscience of vision. Oxford University Press, Oxford
Sailer U, Flanagan JR, Johansson RS (2005) Eye-hand coordination during learning of a novel visuomotor task. J Neurosci 25(39):8833–8842
Seidler RD, Bo J, Anguera JA (2012) Neurocognitive contributions to motor skill learning: the role of working memory. J Mot Behav 44(6):445–453
Sekiyama K, Miyauchi S, Imaruoka T, Egusa H, Tashiro T (2000) Body image as a visuomotor transformation device revealed in adaptation to reversed vision. Nature 407(6802):374–376
Singer W, Tretter F, Yinon U (1982) Central gating of developmental plasticity in kitten visual cortex. J Physiol 324:221–237
Sperry RW (1941) The effect of crossing nerves to antagonistic muscles in the hind limb of the rat. J Comp Neurol 75(1):1–19
Sperry RW (1943a) Effect of 180 degree rotation of the retinal field on visuomotor coordination. J Exp Zool 92(3):263–279
Sperry RW (1943b) Functional results of crossing sensory nerves in the rat. J Comp Neurol 78(1):59–90
Sperry RW (1947) Effect of crossing nerves to antagonistic limb muscles in the monkey. Arch Neurol Psychiatry 58(4):452–473
Stratton GM (1896) Some preliminary experiments on vision without inversion of the retinal image. Psychol Rev 3(6):611
Stratton GM (1897) Vision without inversion of the retinal image. Psychol Rev 4(4):341
Sugita Y (1996) Global plasticity in adult visual cortex following reversal of visual input. Nature 380(6574):523–526
Taylor JA, Ivry RB (2011) Flexible cognitive strategies during motor learning. PLoS Comput Biol 7(3):e1001096
Thoroughman KA, Shadmehr R (2000) Learning of action through adaptive combination of motor primitives. Nature 407(6805):742–747
Todorov E, Jordan MI (2002) Optimal feedback control as a theory of motor coordination. Nat Neurosci 5(11):1226–1235
Welch RB, Choe CS, Heinrich DR (1974) Evidence for a three-component model of prism adaptation. J Exp Psychol 103(4):700–705
Werner S, Bock O (2010) Mechanisms for visuomotor adaptation to left–right reversed vision. Hum Mov Sci 29:172–178
Wolpert DM, Kawato M (1998) Multiple paired forward and inverse models for motor control. Neural Netw 11(7–8):1317–1329
Wolpert DM, Miall RC (1996) Forward models for physiological motor control. Neural Netw 9(8):1265–1279
Acknowledgments
We would like to thank Mohamed N. Abdelghani for conversations relating to the theoretical aspects of this paper. T.P.L. was supported by an Ontario Graduate Scholarship and by the Sun Microsystems of Canada Scholarship in Computational Sciences and Engineering. J.F-R. was supported by UNAM grant PAPIIT IN202810 and CONACYT grant 102314. P.M-B. was supported by a CONACyT award. D.B.T. was supported by CIHR. N.F.T. was supported by NSERC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Timothy P. Lillicrap and Pablo Moreno-Briseño contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lillicrap, T.P., Moreno-Briseño, P., Diaz, R. et al. Adapting to inversion of the visual field: a new twist on an old problem. Exp Brain Res 228, 327–339 (2013). https://doi.org/10.1007/s00221-013-3565-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3565-6