Abstract
Rationale
Deficient response inhibition is a prominent feature of many pathological conditions characterised by impulsive and compulsive behaviour. Clinically effective doses of catecholamine reuptake inhibitors are able to improve such inhibitory deficits as measured by the stop-signal task (SST) in humans and other animals. However, the precise therapeutic mode of action of these compounds in terms of their relative effects on dopamine (DA) and noradrenaline (NA) systems in prefrontal cortical and striatal regions mediating attention and cognitive control remains unclear.
Objectives
We sought to fractionate the effects of global catecholaminergic manipulations on SST performance by using receptor-specific compounds for NA or DA. The results are described in terms of the effects of modulating specific receptor subtypes on various behavioural measures such as response inhibition, perseveration, sustained attention, error monitoring and motivation.
Results
Blockade of α2-adrenoceptors improved sustained attention and response inhibition, whereas α1 and β1/2 adrenergic receptor antagonists disrupted go performance and sustained attention, respectively. No relevant effects were obtained after targeting DA D1, D2 or D4 receptors, while both a D3 receptor agonist and antagonist improved post-error slowing and compulsive nose-poke behaviour, though generally impairing other task measures.
Conclusions
Our results suggest that the use of specific pharmacological agents targeting α2 and β noradrenergic receptors may improve existing treatments for attentional deficits and impulsivity, whereas DA D3 receptors may modulate error monitoring and perseverative behaviour.
Similar content being viewed by others
Introduction
The discovery that drugs increasing catecholamine levels in prefrontal cortex (PFC) improve cognitive and behavioural deficits in disorders characterised by impulsivity (Bradley 1937; Oades 1987) has opened the way for the investigation of the role of dopamine (DA) and noradrenaline (NA) in behavioural inhibition and attention. A key question in the psychopharmacology of impulsive behaviour is whether the effects of anti-impulsivity drugs are mainly mediated by DA, NA or both (de Wit et al. 2002; Eagle et al. 2008; Robbins and Arnsten 2009), although this is not a simple task due to the complex interactions between the two catecholaminergic systems in the PFC (e.g., Antelman and Caggiula 1977; Pan et al. 2004). Noradrenergic projections from the locus coeruleus (LC) and dopaminergic neurons arising from the ventral tegmental area converge in the medial PFC (mPFC; Berger et al. 1974; Lindvall and Bjorklund 1974; Thierry et al. 1973). There, the NA transporter participates in the reuptake of DA (Carboni and Silvagni 2004; Moron et al. 2002; Tanda et al. 1997), compensating for the paucity of dopamine transporter (DAT) sites in this area (Ciliax et al. 1995; Sesack et al. 1998). The main goal of the present investigation is to better define the differential contribution of specific noradrenergic and dopaminergic agents on stop-signal task (SST) performance, which has been used extensively in the assessment of motor impulsivity in humans.
The SST measures the ability to stop an already initiated response as well as the speed of the inhibitory processes (i.e., the stop-signal reaction time, SSRT; Logan 1994). Response inhibition is impaired in several psychiatric disorders characterised by impulsive behaviour (Lipszyc and Schachar 2010), especially in patients with attention deficit/hyperactivity disorder (ADHD; Aron and Poldrack 2005; Schachar et al. 1995; Verbruggen and Logan 2008). Stimulant and non-stimulant ADHD medications include methylphenidate and atomoxetine as the prototypical drugs of these two classes, respectively. Both have comparable efficacy in ADHD (Hazell et al. 2011; van Wyk et al. 2012), although psychostimulants remain widely used for this purpose (Wilens 2008). However, their exact mechanism of action is still unknown. Rodent studies have shown that methylphenidate and atomoxetine increase in vivo extracellular levels of NA and DA in PFC, whereas only methylphenidate increases subcortical DA levels (Bymaster et al. 2002). Thus, the positive effects of atomoxetine and methylphenidate on SSRT may not be mediated exclusively by NA. Results obtained after the administration of various classes of agonist and antagonists at catecholaminergic receptors may contribute in advancing our understanding of the neural substrates and cognitive functions targeted by clinically effective compounds.
Several studies have investigated effects of adrenoceptor agonists and antagonists on attention and impulsivity in both human and non-human subjects. The α2 receptor agonist guanfacine has been proposed as a potential treatment for ADHD and as a useful alternative to psychostimulant medication (Scahill et al. 2001; Taylor and Russo 2001). However, previous studies failed to find any improvement of guanfacine on SST performance in humans (Muller et al. 2005) and rats (Bari et al. 2009). α1-Adrenoceptor agonists improve sustained attention in rats, whereas α1 antagonist administration has the opposite effect and abolishes the positive effects of the agonist (Puumala et al. 1997). Antagonists at the α1 receptor also counteract the beneficial effects of methylphenidate (Berridge et al. 2012) and of the selective NA reuptake inhibitor (SNARI) reboxetine (Liu et al. 2009). In general α1-adrenergic receptors are thought to influence behavioural states and arousal levels in synergy with β-adrenoceptors (Berridge and Espana 2000; Stone and Quartermain 1999). Thus, α2-, α1- and β-adrenoceptors may well be implicated in attention and response control as measured by the SST.
Although previous reports have attributed to DA an important role in behavioural activation, rather than inhibition (Eagle et al. 2008), recent findings have demonstrated a more complex role for dopaminergic neurotransmission during SST performance: blocking D2 receptors in the dorso-medial striatum prolonged SSRT, whereas D1 receptor antagonism in the same area improved stopping (Eagle et al. 2011). Moreover, in humans and other animals, striatal dopamine D2/D3 receptors represent an important link between impulsivity and drug addiction (Caprioli et al. 2013; Dalley et al. 2007; Volkow et al. 2007). Dopamine D3 receptors modulating locomotor activity, and the reinforcing properties of drugs and food (Barik and de Beaurepaire 2005; Caine and Koob 1993; Daly and Waddington 1993; Duarte et al. 2003a; Pilla et al. 1999), are mainly located in the nucleus accumbens, cerebellum, olfactory tubercle and islands of Calleja (Bouthenet et al. 1991; Sokoloff et al. 1990). However, their exact function is not very well understood. Thus, since DA D1- and D2-like receptors may have opposite effects on impulsivity (Pattij et al. 2007; Pezze et al. 2007; van Gaalen et al. 2006), the systemic administration of selective compounds could produce results not observed previously on SST performance. The dopamine D4 receptor gene (DRD4), coding for a G-protein coupled receptor primarily found in cortico-limbic areas (Ariano et al. 1997; Oak et al. 2000), has been one of the most consistently implicated genes in ADHD (Faraone et al. 2001; Holmes et al. 2002; LaHoste et al. 1996; Langley et al. 2004; Smalley et al. 1998). The DRD4 7-allele repeat has been positively associated with novelty-seeking and impulsivity (Colzato et al. 2010; Congdon et al. 2008), and shown to affect prefrontal grey matter volume in normal and ADHD subjects (Durston et al. 2005). Because of the high levels of D4 receptors in PFC and its high affinity for NA (Lanau et al. 1997; Newman-Tancredi et al. 1997), it is conceivable that drugs acting at D4 receptors may play a role in response inhibition as measured by the SST.
To better understand the differential contribution of the compounds tested on SST performance, we analysed several additional measures that were not reported in previous investigations using the rat SST. These include the intra-individual variability of reaction times (SDGoRT) and the post-error slowing (PES) that have been extensively investigated in the human literature and are found to be altered in ADHD, impulsive subjects and several pathological conditions (e.g., Adams et al. 2011; Baldwin et al. 2004; Boonstra et al. 2005; Epstein et al. 2006, 2011; Fitzpatrick et al. 1992; Frank et al. 2007; Jones et al. 2008; Kaiser et al. 2008; Kollins et al. 2008; MacDonald et al. 2009; Nandam et al. 2010; Spencer et al. 2009). High intra-individual variability of reaction times is probably the most replicated and stable finding in children with ADHD (Russell et al. 2006) and is considered diagnostic of ‘lapses of attention’ (Castellanos et al. 2006; Leth-Steensen et al. 2000). Although relatively few studies have reported SDGoRT in rats, reaction time variability seems to predict attentional performance in both normal animals and animals made distractible by experimental manipulations (Hausknecht et al. 2005; Loos et al. 2012; Narayanan et al. 2006). On the other hand, PES depends on the ability of the subject to adjust ongoing performance on the basis of negative feedback (Rabbitt 1966). PES is thus regarded as a measure of performance monitoring (Botvinick et al. 2001; Kerns et al. 2004) and of the ability to dynamically implement cognitive control over one's behaviour (Gilmour et al. 2012). On trials following stop errors, normal subjects display slower reaction times as an attempt to improve performance. Comparing reaction times before and after a stop error in rats performing the SST produces a measure that is sensitive to pharmacological manipulations, partly confirming its construct validity. Other secondary variables reported that are specific to rodent behavioural testing are the reward collection latency (RCL) and nose-poke perseveration during TO periods (NP/TO), putative measures of motivation and compulsive behaviour, respectively.
Materials and methods
Subjects were male Lister Hooded rats purchased from Charles River, UK, in all the experiments. Rats were housed in groups of four, under a reversed 12:12-h light–dark cycle (lights off at 07:30), and were tested during the dark phase of this cycle. For behavioural training and testing, rats were food-restricted and maintained at 85 % of their free-feeding body weight feeding them 15 g of standard laboratory chow (Purina Rat Chow) on rest days and 10 g on SST days plus reinforcer pellets (Test Diet, 45 mg precision-weight, purified ingredient rodent tablets, Sandown Scientific). Water was freely available except during testing. All experiments were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986.
Behavioural training
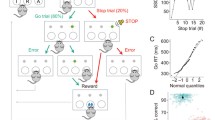
Rats were trained following a procedure modified from Eagle and Robbins (2003) and written in Visual Basic by A. C. Mar to perform the SST. Subjects were first habituated to the testing apparatus where they learned to collect free pellets from the food well. On the next day, rats were presented with the right lever extended into the box and gradually learned to press it to receive a reward pellet into the food well. Collection of the reward started the subsequent trial with the right lever re-introduced into the box. When the animals reliably completed a session of 100 trials within 30 min on 2 consecutive days, they were presented with the left lever and learned to press it to extend the right one, which will result in the delivery of the reward if pressed within 30 s. The limited hold (LH) — the time available for the rats to press the right lever after pressing the left one — was progressively shortened until the rats reliably completed 100 trials with an LH of 5 s. Stop trials were then introduced using a stop-signal tone (4,500 Hz, ~80 dB) that lasted until the end of the LH period and the number of total trials was set to 210, to be completed within 30 min. The LH and stop-signal duration were made gradually shorter over several sessions until they were kept constant for each animal (final LH was 1.2 s). The tone length was further shortened until it reached 200 ms. For all sessions, the task was initiated when the rats nose-poked into the central food well. During go trials the rats were rewarded with a food pellet for pressing the left then the right lever in sequence before the LH ended. If the rats failed to press the right lever within the LH, they received a time-out period (TO; 5 s darkness, no levers available) and the trial was recorded as a go error. The latency of the go response (go reaction time [GoRT]) is the time elapsed from the left to the right lever presses (Fig. 1).
Schematic illustration of the SST. A standard session consists of 210 trials to be completed within 30 min. On 20 % of the trials (stop trials), a stop-signal will be played after the left lever has been pressed and after a variable stop-signal delay (SSD), which is based on the mean reaction time (mRT) of the subjects on previous sessions: zero delay (ZD), mRT −350 or mRT −150 ms. The stop-signal instructs the animal that the go response to the right lever has to be inhibited in order to obtain the reward. On the remaining 80 % of the trials (go trials), the left and right levers have to be pressed in rapid sequence and the go reaction time (GoRT) has to be shorter than the limited hold (LH; 1.2 s) in order to receive a reward, which is delivered in the central food well (picture modified from Bari et al. 2011)
Stop trials were delivered pseudo-randomly on 20 % of total trials. Stop trials began in the same manner as a go trial, but after pressing the left lever, the stop-signal was played and animals were rewarded if they refrained from pressing the right lever for the duration of the LH. If the rats pressed the right lever after the stop-signal was played, they were punished with a TO, but if they pressed the right lever before the occurrence of a stop-signal in a stop trial, that trial was re-classified as a go trial. During training, stop-signals were played as soon as the rats pressed the left lever (zero delay [ZD]), whereas during baseline and testing sessions stop-signals were played at a pre-determined delay (stop-signal delay [SSD]). Four different SSDs were used (mean GoRT [mRT]: −350, −250, −150, and −50 ms) plus ZD pseudo-randomly interspersed among go trials in order to draw the baseline inhibition function (Logan 1994). For test sessions, two SSDs were used and were calculated from the mRTs averaged from three previous baseline sessions at ZD, and these were individual mRTs −350 and −150 ms for all the experiments. Rats were excluded from the experiment if they displayed one of the following characteristics during baseline sessions: (1) inverted inhibition function (i.e., better stop accuracy with longer SSDs); (2) too low or too high average stop accuracy (not within the 20–80 % range; Band et al. 2003); (3) go accuracy below 80 %. Given the complexity of the task and the elevated number of assumptions required by the model to be met by the subjects in order to reliably calculate the SSRT, a relatively high number of animals has to be excluded from the final data analysis, a problem encountered also in experiments with human subjects (e.g., Castellanos and Tannock 2002; Solanto et al. 2001).
SSRT calculation
SSRTs were estimated using the ‘race model’ protocol described by Logan (1994). Briefly, GoRTs were rank-ordered for each SSD and the nth GoRT was selected from the ranked list. The n value was obtained by multiplying the number of GoRTs in the distribution by the probability of responding on stop trials at one given SSD. To obtain the SSRT, the respective SSDs were subtracted from the nth GoRT. SSRTs were then averaged to give a single estimate for each rat for each test session. SSRT and stop accuracy (i.e., percent of stop trials in which the go response was correctly inhibited) were adjusted for the presence of omission errors on go trials (go errors) in order to correct for the stop trials when an inhibition could not be attributed to a successful stop, but could be accounted for by distraction or inattention. In other words, this procedure adjusts for those successful stop trials where the animals would not have completed the go response whatever the trial type (go or stop). Adjustment was performed using the correction factor of Tannock et al. (1989): adjusted p (inhibit) = observed p (inhibit) − p (omission)/1 − p (omission), where p (inhibit) is the stop accuracy and p (omission) is 1 − go accuracy, expressed as ratios.
Secondary variables
Dependent variables analyzed in the following experiments include mRT (the latency of the go response averaged over the number of correct go trials), stop accuracy (presented as a percentage of total stop trials) and go accuracy (presented as a percentage of total go trials).
Some additional measures to those previously described for the rat SST were analysed: the within-subject standard deviation of reaction times during go trials (SDGoRT), which is considered diagnostic of ‘lapses of attention’ (Castellanos et al. 2006; Leth-Steensen et al. 2000) or of an inability to sustain stimulus–response contingencies (Picton et al. 2007). PES, which is derived from the difference between GoRTs on trials immediately after, and GoRTs on trials immediately before, a stop error. This latter variable is considered as a measure of performance monitoring/adjustment (Gehring et al. 1993; Li et al. 2006b; Schachar et al. 2004) in the human literature but, since rats usually show a decrease in GoRT after a failed stop trial, it is usually a negative value (see discussion). A significant change in PES in the experiments here described is interpreted as a change in the capacity of the animal to use errors to guide subsequent behaviour and/or as a variation in speed–accuracy trade-off strategy. Finally, the number of nose-pokes made into the food well during TO periods (total nose-pokes divided by the total number of TO periods; NP/TO), thus when there is no programmed consequence for this action, is considered as a measure of perseveration and the latency to collect the reward from the food well (RCL) is interpreted as a measure of motivation.
Drugs
Drug doses were adapted from available published data or chosen from previous dose–response curve experiments and published functional neurochemistry data. Solutions were freshly prepared every day. Different groups of animals were used for each drug and at least 2 days were allowed between drug injections. During the time between the administration of the compound and the beginning of the task, animals where singly housed in holding cages and left undisturbed in a quiet room. All drugs were administered via intraperitoneal injections at a volume of 1 ml/kg and according to a randomized Latin square design, unless otherwise stated.
Atipamezole (α2 adrenoceptor antagonist)
A group of 14 animals (350–400 g) were injected with the highly selective α2 antagonist atipamezole (Pertovaara et al. 2005; Antisedan, Pfizer). Atipamezole (0.03, 0.1, 0.3 mg/kg, plus vehicle) was diluted in 0.9 % saline and administered 45 min before test sessions (Haapalinna et al. 1998; Scheinin et al. 1988; Sirvio et al. 1993; Virtanen et al. 1989). Three animals were excluded from the final analysis for violation of the race model assumptions (final n = 11).
Prazosin (α1 adrenoceptor antagonist)
Fourteen subjects weighing between 370 and 450 g were administered prazosin (0.05, 0.15, 0.5 mg/kg, plus vehicle), which was dissolved in double distilled water (DDW) and administered at a volume of 2 ml/kg, 45 min before test sessions. Drug doses were chosen based on published studies (e.g., Darracq et al. 1998; Selken and Nichols 2007). Two subjects have been excluded from the final analysis in this study because of violation of the race model assumptions (final n = 12).
Propranolol (β 1/2 adrenoceptor antagonist)
A group of fourteen animals (370–470 g) was administered propranolol (0.3, 1.5, 3 mg/kg, plus vehicle) which was dissolved in DDW and injected 45 min before test sessions (Hahn and Stolerman 2005). Three subjects violated the race model assumptions and were excluded (final n = 11).
DA D1 and D2 receptor antagonists
Two groups of eighteen animals (320–440 g) were administered the selective DA D1 receptor antagonist SCH-23390 (Sidhu et al. 1986) and the DA D2/3 receptor antagonist sulpiride, both purchased from Sigma-Aldrich. For sulpiride, doses were 1, 5, 10 mg/kg, plus vehicle (0.9 % saline) injected 45 min before test and the solution adjusted with hydrochloric acid to give a pH of ~6 (Lacroix et al. 2003; Passetti et al. 2003; Sorge and Clarke 2009). SCH-23390 doses were 1, 5, 10 μg/kg, plus vehicle (0.9 % saline) administered 45 min before testing (Koffarnus et al. 2011; van Gaalen et al. 2006). Five animals from each group were excluded from the final analysis, because of violation of the race model assumptions (final n = 13 + 13).
DA D3 receptor agonist and antagonist
Two groups of twenty two rats weighing between 320 and 450 g were administered the DA D3 receptor-preferring agonist 7-OH-PIPAT or the antagonist nafadotride both purchased from Tocris (Bristol, UK). Doses were: nafadotride, 0.3, 1, 3 mg/kg, plus saline; 7-OH-PIPAT, 0.1, 0.3, 1 mg/kg, plus saline (Flietstra and Levant 1998; Khroyan et al. 1997; Levant and Vansell 1997). Both drugs were injected 30 min before test sessions. Six rats from the nafadotride experiment (final n = 16) and three from the 7-OH-PIPAT one (final n = 19) were excluded from the final analysis for not performing according to the requirements of the SST.
DA D4 receptor agonist and antagonist
Two groups of 15 rats weighing between 360 and 470 g received the DA D4 selective agonist PD 168,077 or the DA D4 selective antagonist l-745,870. These compounds were purchased from Tocris (Bristol, UK) and dissolved in 0.9 % saline solution and 20 % β-hydroxypropyl-cyclodextrin, respectively. Doses were 0.5, 1, 5 mg/kg, plus vehicle, for both drugs (Koffarnus et al. 2011; Nayak and Cassaday 2003). Five rats from each group were excluded from the final statistical analysis because of violation of the race model assumptions (final n = 10 + 10).
Data analysis
Repeated measure ANOVA was used for all the experiments with drug dose level as a within-subjects factor and Sidak's post-hoc adjustment for multiple comparisons was applied if a main effect was found. Mauchly’s test of sphericity was used and Huynh-Feldt corrected degrees of freedom rounded to the nearest integer are presented when the assumption of homogeneity of covariance was violated. All tests of significance were performed at α = 0.05. Graphs and tables display means and their standard errors (SEM); asterisks indicate significance at the level of p < 0.05 (*) or p < 0.01 (**).
Results
Effects of atipamezole
Atipamezole (Fig. 2) significantly affected SSRT (F (3,30) = 3.09, p < 0.05). Post-hoc tests showed that SSRT was decreased (i.e., speeded) at 0.3 mg/kg compared with the vehicle condition (p < 0.05). ANOVA also revealed a main effect of the drug on mRT (F (3,30) = 3.46, p < 0.05). However, Sidak corrected post-hoc analyses did not show any significant differences between doses. No significant effect (ns) was detected for stop accuracy (F (3,30) = 0.43, ns), go accuracy (F (3,30) = 0.74, ns), PES (F (1,14) = 1.9, ns), NP/TO (F (2,18) = 2.64, ns) and RCL (F(2,24) = 0.71, ns). There was a significant main effect of the drug on SDGoRT (F (3,30) = 5.16, p < 0.01; Table 1). According to pairwise comparisons the highest dose (0.3 mg/kg) significantly decreased SDGoRT compared to vehicle (p < 0.05).
Effects of prazosin
Prazosin administration did not affect SSRT (F (3,33) = 0.61, ns; Fig. 3). ANOVA revealed a main effect of the drug on mRT (F (3,33) = 10.66, p < 0.01). Both 0.15 mg/kg (p < 0.05) and 0.5 mg/kg (p < 0.01) increased mRT compared with vehicle, according to post-hoc analyses. Stop accuracy was not affected (F (3,33) = 1.44, ns), but there was a significant main effect on go accuracy (F(3,33) = 8.9, p < 0.01). Post-hoc analyses revealed that at 0.5 mg/kg go accuracy was significantly lower compared with vehicle (p < 0.01) and 0.05 mg/kg (p < 0.05). SDGoRT was also significantly affected by prazosin administration (F (3,33) = 3.26, p < 0.05). Pairwise comparisons, however, showed no significant differences after Sidak’s correction. There was no difference regarding PES (F (1,14) = 0.55, ns) and RCL (F (1,16) = 2.31, ns), while a significant difference was found for NP/TO (F (3,33) = 6.13, p < 0.01; Table 1). Pairwise comparisons showed that only at 0.05 mg/kg animals made fewer perseverative nose pokes (NP/TO) into the food well during time-out periods compared with the vehicle condition (p < 0.05).
Effects of propranolol
There was no effect of propranolol on SSRT (F (3,30) = 2.16, ns; Fig. 4) and stop accuracy (F (3,30) = 0.05, ns). mRT was significantly affected by the drug (F (3,30) = 3.41, p < 0.05), but pairwise comparisons reported no significant differences between doses. There was also a significant main effect on go accuracy (F (3,30) = 3.51, p < 0.05), but no significant differences after correcting for multiple comparisons. Propranolol significantly affected SDGoRT (F (3,30) = 3.82, p < 0.05; Table 1) and pairwise comparisons showed that it was higher after the 3 mg/kg dose, compared with the vehicle condition (p < 0.05). There was no effect on PES (F (3,26) = 0.3, ns), NP/TO (F (3,30) = 1.63, ns) and RCL (F (2,20) = 0.31, ns).
Effects of D1 and D2 receptor antagonists
SCH-23390 (Fig. 5 and Table 2) had no significant effect on SSRT (F (3,36) = 1.1, ns), mRT (F (3,36) = 0.94, ns), stop accuracy (F (3,36) = 1.81, ns), go accuracy (F (3,36) = 1.29, ns), SDGoRT (F (3,36) = 1.33, ns), NP/TO (F (1,13) = 1.96, ns), PES (F (3,36) = 0.73, ns) or RCL (F (3,36) = 0.67, ns).
Sulpiride administration (Fig. 6 and Table 2) did not affect SSRT (F (3,36) = 1.49, ns), mRT (F (3,36) = 1.23, ns) or go accuracy (F (3,36) = 1.58, ns). There was a significant main effect to impair stop accuracy (F (3,36) = 3.0, p < 0.05). Pairwise comparisons showed that at 10 mg/kg the animals displayed higher stop accuracy compared to 5 mg/kg. SDGoRT was not changed by sulpiride administration (F (3,36) = 1.3, ns) and PES (F (3,36) = 0.71, ns) and NP/TO (F (2,22) = 1.38, ns) were also left unchanged. There was a trend towards a significant effect on RCL (F (3,36) = 2.75, p = 0.057).
Effects of DA D3 receptor agonist and antagonist
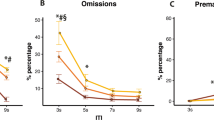
7-OH-PIPAT administration (Fig. 7) had no effect on SSRT (F (3,54) = 1.17, ns). The drug, however, had a strong effect to slow mRT (F (3,54) = 31.24, p < 0.01). Pairwise analyses showed that all doses slowed mRT compared with vehicle (p < 0.01) and that at the highest dose (1 mg/kg) mRT was slower compared with all the other conditions (p < 0.01). 7-OH-PIPAT also affected stop accuracy (F (3,54) = 3.10, p < 0.05), but pairwise comparisons did not detect significant differences between doses. Repeated measures ANOVA showed that 7-OH-PIPAT administration significantly affected go accuracy (F (2,30) = 34.11, p < 0.01). According to post-hoc pairwise comparisons, 1 mg/kg impaired go accuracy compared with all the other conditions (p < 0.01), 0.3 mg/kg also being different from the vehicle condition (p < 0.01). There was no effect of the drug on SDGoRT (F (2,42) = 0.77, ns), but a main effect was detected for PES (F (2,36) = 7.31, p < 0.01; Table 2). In this case, the highest dose (1 mg/kg) increased PES compared with the vehicle (p < 0.01) and the 0.1 mg/kg (p < 0.05) conditions. Also, 0.3 mg/kg increased PES compared with 0.1 mg/kg (p < 0.05), but not compared with vehicle. ANOVA revealed a significant effect on NP/TO (F (2,31) = 4.25, p < 0.05) and RCL (F (2,41) = 3.87, p < 0.05); only in this latter case, pairwise comparisons showed that the highest dose (1 mg/kg) slowed the rats compared with the vehicle condition (p < 0.05), but failed to find a significant difference between doses for NP/TO.
Administration of the dopamine D3 receptor agonist 7-OH-PIPAT produced strong detrimental effects, specifically on go measures. mRT was longer than vehicle at all the doses tested (p < 0.01) and the highest dose was also different from all the other conditions (p < 0.01). Go accuracy was lower at 1 mg/kg (p < 0.01 compared with all the other conditions) and at 0.3 mg/kg (p < 0.01) compared with vehicle (Veh). **p < 0.01
Repeated-measures ANOVA revealed a main effect of nafadotride on SSRT (F (3,45) = 3.49, p < 0.05; Fig. 8); pairwise comparisons showed that the highest dose (3 mg/kg) significantly slowed SSRT compared with vehicle controls (p < 0.05). A main effect of the drug on mRT was also found (F(2,35) = 11.78, p < 0.01). Post-hoc analyses revealed that 3 mg/kg of nafadotride slowed mRT compared with both vehicle (p < 0.01) and 0.3 mg/kg (p < 0.01), while mRT at 1 mg/kg was slower only compared with the 0.3 mg/kg dose (p < 0.05). No effects of nafadotride were detected on stop accuracy (F (3,45) = 2.26, ns) and SDGoRT (F (3,45) = 2.58, p = 0.065, ns). Go accuracy was affected by the drug (F (1,22) = 21.57, p < 0.01) only at the highest dose (3 mg/kg) at which it was lower compared with all the other conditions (p < 0.01). There was a main effect of the drug on PES (F (3,45) = 5.39, p < 0.01; Table 2), with the highest dose (3 mg/kg) making the animals significantly slower after a stop error (p < 0.05). ANOVA revealed a significant effect of the drug on NP/TO (F (3,45) = 13.7, p < 0.01), but not on RCL (F (2,24) = 0.015, ns). Pairwise comparisons showed that, at the highest dose (3 mg/kg), NP/TO was significantly lower than in all the other conditions (p < 0.05).
Nafadotride (D3 antagonist) significantly increased SSRT at 3 mg/kg compared with vehicle administration (p < 0.05). The same dose produced longer mRT (p < 0.01 compared with vehicle) and lower go accuracy (p < 0.01 compared with all the other conditions). At the dose of 0.3 mg/kg mRT was faster than both 1 (p < 0.05) and 3 mg/kg (p < 0.01). *p < 0.05; **p < 0.01
Effects of DA D4 agonist and antagonist
There was a main effect of PD-168,077 to prolong SSRT (F (3,27) = 4.92, p < 0.01; Fig. 9). Pairwise comparisons showed that 5 mg/kg significantly increased SSRT compared with 0.5 mg/kg (p < 0.05). PD-168,077 administration did not significantly influence mRT (F (3,27) = 2.88, p = 0.054, ns), stop accuracy (F (2,17) = 0.77, ns) or go accuracy (F (2,14) = 1.08, ns). SDGoRT (F (2,14) = 0.17, ns) and PES (F (3,27) = 1.29. ns) were also not affected by the drug at any of the doses tested (Table 2). There was no significant effect on RCL (F (2,27) = 0.25, ns) or NP/TO (F (1,19) = 0.11, ns).
There was no effect of l-745,870 on SSRT (F (3,27) = 0.76, ns; Fig. 10), mRT (F (3,27) = 1.92, ns), stop accuracy (F (3,27) = 0.81, ns), SDGoRT (F (3,27) = 0.25, ns) or PES (F (3,27) = 0.76, ns). There was a main effect of the drug on go accuracy (F (3,27) = 8.02, p < 0.01) and post-hoc analyses showed that the highest dose (5 mg/kg) impaired go accuracy compared with vehicle and 0.5 mg/kg (p < 0.05). There was no effect of the drug on NP/TO (F (1,21) = 0.45, ns) or RCL (F (2,35) = 0.092, ns; Table 2).
Discussion
We aimed to characterise mechanisms underlying the beneficial effects on SST performance in rats of catecholamine reuptake blockers and other agents used for the treatment of disorders such as ADHD that exhibit impulsive behaviour. Commonly used stimulant and non-stimulant medications act globally on the catecholaminergic systems and that lead to unwanted side effects as well as preventing the formulation of hypotheses regarding the mechanisms by which these drugs affect specific executive functions. To parcel out the contribution of different receptors, we also analysed secondary variables of the SST for rats that are commonly reported in experiments with human subjects. Variations of these measures in response to specific receptor activation or blockade can aid the interpretation of the standard SST measures, the comparison with human data and the understanding of the underlying cognitive processes affected by therapeutic drugs acting as so-called cognitive enhancers.
Effects of noradrenergic ligands
We showed that atipamezole, a very selective and potent α2-adrenergic receptor antagonist (Haapalinna et al. 1997; Virtanen 1989), speeded SSRT at the 0.3 mg/kg dose and decreased response variability (i.e., improved sustained attention). From the present results, it seems that the speeding of inhibitory processes and the improved sustained attention after atipamezole administration are not necessarily causally related since stop accuracy was not significantly affected by this drug. This pattern of results apparently contrasts with previous evidence of a deleterious effect of the less selective α2 receptor antagonists idazoxan and yohimbine on attention and impulsivity (Arnsten and Li 2005; Rowe et al. 1996; Sun et al. 2010; Swann et al. 2005, 2013). However, these results might be non-specific to α2 receptor antagonism because the more selective drug atipamezole improves attention and other cognitive functions (Devauges and Sara 1990; Haapalinna et al. 1998; Lapiz and Morilak 2006; Mervaala et al. 1993; Pertovaara et al. 2005), consistent with the present findings. Alternatively, the different attentional and inhibitory requirements of the SST, compared to other behavioural tasks, may be the reason for this discrepancy. On the other hand, α2 receptor agonist administration has deleterious consequences on attention (Smith and Nutt 1996) and target detection (Brown et al. 2012; Coull et al. 2004) in some studies, but positive effects in others (Fernando et al. 2012). Thus, a better understanding of the effects of drugs acting directly at the noradrenergic α2 receptor requires a more specific definition of the cognitive construct measured as well as the knowledge of the α2 receptor subtype affected by the drug.
Atipamezole’s positive effects on cognitive tasks are thought to be mediated mainly through its actions on pre-synaptic α2 receptors to which it preferentially binds at low doses, while post-synaptic α2 receptors have been implicated in the memory improvements seen after α2 receptor agonist administration (Ji et al. 2008), especially in animals with memory impairments (Arnsten and Cai 1993; Arnsten et al. 1988; Berridge et al. 1993; Franowicz and Arnsten 1998; Rama et al. 1996). Similarly, the beneficial effects of atipamezole on cognition are most reliably seen in aged or poor performing subjects, or in situations of increased attentional demand (Coull et al. 1996; Haapalinna et al. 1998, 2000; Jakala et al. 1992; Sirvio et al. 1993). These baseline-dependent effects are consistent with the finding that atipamezole increases NA turnover rate significantly more in the brains of aged than that of young adult rats (Haapalinna et al. 2000), suggesting that it acts by restoring optimal levels of noradrenergic transmission. These results, however, do not rule out the possibility that atipamezole’s positive effects are mediated also by DA, since this and other α2-adrenergic receptor antagonists increase DA release in the rat mPFC (Devoto et al. 2001; Gobert et al. 1997; Gresch et al. 1995; Matsumoto et al. 1998; Yamamoto and Novotney 1998), possibly via indirect activation of α1 receptors (Anden et al. 1982).
Blocking α1-adrenergic receptors by prazosin increased mRT and decreased go accuracy and NP/TO, consistent with a mild sedative effect of this drug (Berridge and Espana 2000) and, more generally, with the role of α1 adrenoceptors in locomotor activity and arousal (Sirvio and MacDonald 1999). Prazosin inhibits the electrically or pharmacologically-evoked release of DA in the nucleus accumbens and PFC, as well as the locomotor enhancing effects of amphetamine and cocaine (Darracq et al. 1998; Drouin et al. 2002; Gioanni et al. 1998). These secondary effects of prazosin on the dopaminergic system are consistent with the disruptive effects on go performance observed here. Moreover, since mRT and go accuracy are often considered as secondary measures of sustained attention (Castellanos and Tannock 2002; Lijffijt et al. 2005, 2006; Overtoom et al. 2002), the effects of prazosin on SST performance are indicative of detrimental effects on attention but not impulsivity, in keeping with previous results on five-choice serial reaction time task (5-CSRTT) performance (Hahn and Stolerman 2005; Puumala et al. 1997).
The effect of propranolol administration on SST variables was similar to that of prazosin. Both drugs mainly affected go performance, although for propranolol the effects were not significant after adjusting for multiple comparisons. Propranolol blocks both β1- and β2-adrenergic receptors (Sibley et al. 1986) and has been shown to impair attentional performance in humans (De Martino et al. 2008; Strange and Dolan 2007) and rats (Hahn and Stolerman 2005), which is consistent with the increase in response variability (SDGoRT) observed in the present study. Recently, Pattij and co-workers (2012) have shown that selective β1 and β2 adrenoceptor agonists improve attention and impulsivity in the 5-CSRTT (Bari et al. 2008; Robbins 2002). These results are consistent with the evidence that methylphenidate-induced premature responses in the 5-CSRTT can be abolished by co-administration of the β1/2 antagonist propranolol (Milstein et al. 2010). Taken together with the present results, this evidence confirms the important contribution of both α1 and β-adrenergic receptors in attentional processes and stimulus detection.
The results described above are consistent with the suggestion that NA acts post-synaptically to enhance stimulus-evoked neural responsiveness and to regulate tonic spontaneous firing during attentional tasks (Aston-Jones et al. 2000; Berridge and Waterhouse 2003). The positive effects of atomoxetine (Robinson et al. 2008) and atipamezole (present experiment) on SST performance, point to a beneficial role of increasing NA neurotransmission in forebrain areas, although achieved by different mechanisms. NA reuptake blockers like atomoxetine increase extrasynaptic NA content which in turn decreases spontaneous noradrenergic system activity through α2-adrenoceptor stimulation at the level of the LC (Bari and Aston-Jones 2013; De Sarro et al. 1987; Fernandez-Pastor et al. 2005; Grandoso et al. 2004; Szabo and Blier 2001), while atipamezole increases prefrontal NA release by disrupting the feedback inhibitory mechanism (Gobert et al. 1997). These differences are reflected in the behavioural performance of the animals on the SST, with atomoxetine causing an increase in mRT and Go accuracy in addition to its SSRT-speeding effects (Bari et al. 2009), whereas atipamezole is devoid of sedative effects at functionally relevant doses (present results). The motor slowing effects of atomoxetine reflect indirect activation of inhibitory α2 autoreceptors, while the improvements in go accuracy may possibly be due to indirect activation of α1 adrenoceptors. In summary, the advantages of enhancing noradrenergic neurotransmission via α2 receptor antagonism rather than blocking NA reuptake are at least twofold: it prevents (1) the α2 pre-synaptic autoreceptor-mediated negative feedback on NA activity (Gobert et al. 1997) and (2) the post-synaptic α2 receptor-mediated decrease in stimulus-evoked neural responsiveness (Carr et al. 2007; Ji et al. 2008) (Table 3).
Effects of dopaminergic ligands
From the results obtained after SCH-23390 or sulpiride administration, at least at the doses used here, it seems that blocking DA D1 or D2 receptors separately does not influence SST performance. In keeping with the present results, systemic administration of the mixed D1/D2 DA receptor antagonist cis-flupenthixol did not alter SST performance up to doses that impaired the ability of the animals to complete the task and also failed to antagonise the beneficial effects of methylphenidate or modafinil (Eagle et al. 2007). We chose low dose levels for the drugs used in the present experiments in order to preserve the receptor specificity of the compound tested, although it is possible that these doses were too low for SCH-23390 and sulpiride to elicit significant behavioural effects on SST performance. However, previous studies have found significant effects on impulsivity in the 5-CSRTT after 10 μg/kg of SCH-23390 (Koskinen and Sirvio 2001; van Gaalen et al. 2006) and increased risk-aversion after 5 μg/kg (St Onge and Floresco 2009).
On the other hand, sulpiride is known to preferentially affect PFC DA receptors at low doses (Bowers 1984; Kaneno et al. 2001; Kaneno et al. 1991; Kohler et al. 1981; Scatton 1977; Thierry et al. 1986) and to cause place aversion at doses as low as 1 mg/kg (Karami and Zarrindast 2008). Higher doses than the ones used in the present investigation may have produced spurious impairments on SST performance by negatively affecting locomotor activity and incentive motivation. For instance, sulpiride infused directly into the dorso-medial striatum generally impairs SST performance in rats (Eagle et al. 2011), but does not affect 5-CSRTT performance when infused in the mPFC (Granon et al. 2000). Finally, the near-significant effect of sulpiride on RCL is consistent with the increased motivation for food caused by low doses of this drug (Guyon et al. 1993).
The DA D3-preferring agonist 7-OH-PIPAT selectively and negatively influenced motor- and motivation-related measures, without significantly affecting stop-related variables. 7-OH-PIPAT slowed mRT, RCL and decreased go accuracy, consistent with published reports on its strong effects on locomotor activity (e.g., Khroyan et al. 1997). On the other hand, nafadotride administration slowed SSRT and mRT, and decreased go accuracy and NP/TO at 3 mg/kg. The detrimental effects of nafadotride at doses higher than 1 mg/kg are in agreement with the strong cataleptic effect of this drug (Sautel et al. 1995). Nafadotride displays greater selectivity for D3 over D2 receptors in vivo only at doses below ~3 mg/kg when administered via intraperitoneal injection (Levant and Vansell 1997). Thus, since the effects observed in the present experiment are significantly different from the control condition only at 3 mg/kg, it is possible that they are partly due to the drug’s action on D2 receptors. Both nafadotride and 7-OH-PIPAT increased performance monitoring/adjustment as measured by PES, which may be mediated by the mesolimbic DA system where D3 receptors are located (Sokoloff et al. 1990; Stanwood et al. 2000). Although all the behavioural effects of D3 ligands arose in a context of psychomotor depression, the increase in PES cannot be readily assimilated to motor impairments for the way this variable is calculated. However, for both compounds, the beneficial effects on performance control or compulsive nose-poking did not translate in improved stopping. The relatively similar effects produced by administration of D3-preferring agonist and antagonist are puzzling, but not surprising. For instance, both agonist (Duarte et al. 2003b) and antagonist (Vorel et al. 2002) have been shown to attenuate cocaine-induced conditioned place preference. Finally, the similarity of the behavioural effects elicited by nafadotride and 7-OH-PIPAT may be due to the characteristic biphasic dose–effect relationship exhibited by D3 ligands on motivated behaviour (e.g., Depoortere et al. 1996, 1999; Khroyan et al. 1997).
DRD4 knock-out mice do not show enhanced levels of impulsivity in delay discounting and go/no-go tasks. However, these animals display enhanced novelty-seeking behaviour (Helms et al. 2008) and impaired response inhibition in the murine version of the continuous performance task (Young et al. 2011). Contrary to expectations, in the present experiments D4 receptor ligands were ineffective on most SST measures. PD-168,077 mildly slowed SSRT and l-745,870 impaired go accuracy at the higher dose tested. Our results concur with the finding that the presence of a DRD4 polymorphism (7-repeat allele) in children with ADHD does not influence inhibitory processes as measured by the go/no-go task and SST, although these subjects display faster and less accurate response style in neuropsychological tasks compared to non carriers (Langley et al. 2004). Together, these results suggest that the modulation of impulsive behaviour by D4 receptors may depend on the long term effects of their reduced function in the DRD4 7-repeat allele carriers and on the pre-existing state of the dopaminergic and noradrenergic systems. Acute treatment with dopaminergic compounds targeting the D4 receptor may be insufficient in altering inhibitory performance in normal subjects. Baseline-dependent effects have been described for tasks depending on fronto-striatal circuitry in rodents (Milstein et al. 2010; Zhang et al. 2002, 2004) and primates (Arnsten et al. 2000; Jentsch et al. 1999) after administration of D4 antagonists, suggesting that D4 modulation may normalize naturally or chemically altered levels of catecholamines in the PFC. The present study did not take into account baseline differences in performance on response inhibition and is thus not suited to detect such effects. However, future studies will need to investigate the effects of chronic administration of D4-targeting drugs as well as a wider range of doses of the D4 agonist PD-168,077, which had a biphasic effect on SSRT and may have stronger effects at very low doses (e.g., Nayak and Cassaday 2003) (Table 4).
Relevance of secondary SST variables
Here we showed, for the first time in the rat, a dissociation between SSRT and SDGoRT measures; the first assessing the speed of the inhibitory processes (Logan 1994) and the second the intra-individual variability of the go response (Tannock et al. 1995; Teicher et al. 2004). Both measures represent potential endophenotypes to be used as ‘biomarkers’ (Gottesman and Gould 2003; Rommelse et al. 2008) that would help the diagnosis and treatment of clinical disorders such as ADHD and schizophrenia (Castellanos and Tannock 2002; Gilmour et al. 2012; Vaurio et al. 2009). In the present experiments, systemically administered atipamezole improved performance on both SSRT and SDGoRT, whereas propranolol, and to a lesser extent prazosin, selectively impaired SDGoRT. These data complement previous reports on the efficacy of psychostimulants in modulating the trial-to-trial variability of the go response (Baldwin et al. 2004; Boonstra et al. 2005; Epstein et al. 2006, 2011; Fitzpatrick et al. 1992; Nandam et al. 2010; Spencer et al. 2009) and point to a possible involvement of noradrenergic neurotransmission in this behavioural measure (Frank et al. 2007; Kollins et al. 2008; Lee et al. 2010).
In rats, the intra-individual variability of reaction times has been previously shown to increase following various manipulations that cause distractibility, such as distractors presented during reaction time tasks, PFC inactivation and pre-natal alcohol intoxication (Hausknecht et al. 2005; Narayanan et al. 2006). Experimental manipulations known to decrease reaction time variability in rodents are increased stimulus salience and, like in humans, stimulant administration (Sabol et al. 2003). Rodent models of ADHD, such as the spontaneously hypertensive rat, also display highly variable reaction times (Perry et al. 2010b), which suggests a genetic origin for this behavioural trait (Loos et al. 2012; Perry et al. 2010a). Few studies have investigated the relationship between reaction time variability and performance in the 5-CSRTT. Loos et al. (2012) reported a strong correlation between response accuracy (the main attentional measure of the 5-CSRTT) and intra-individual response variability in mice, which is consistent with findings in humans performing an analogous attentional task (Bidwell et al. 2007; Klein et al. 2006). They also identified a quantitative trait locus in common for the two attentional measures on chromosome 16, suggesting that response accuracy and response variability in the 5-CSRTT share underlying genetic origins.
In contrast to human subjects and for rats performing other tasks, rats in the SST show a speeding of GoRT after a stop error. One possible reason for this discrepancy may be the presence of the TO period after a stop error, since it is known that PES decreases as a function of the inter-trial (Rabbitt and Rodgers 1977), or the response–stimulus (Danielmeier and Ullsperger 2011; Jentzsch and Dudschig 2009) interval. Alternatively, subjects may perceive the probability of occurrence of two consecutive stop trials to be low, or they may simply respond impulsively (i.e., faster) after having received punishment (5 s TO). In future studies, eliminating the TO period or varying the percentage of stop trials in a session could help to elucidate the differences in PES between humans and rats performing the SST. Here we considered a positive departure from this baseline post-error ‘speeding’ as an improvement in the capacity of the animal to dynamically adjust ongoing behaviour in order to increase stop accuracy.
Error monitoring, conflict detection and the subsequent adjustment of performance are known to depend on the dorso-medial PFC in humans and rats (Brown and Braver 2005; Chevrier et al. 2007; Falkenstein et al. 2000; Kerns et al. 2004; Li et al. 2008; Modirrousta and Fellows 2008; Narayanan and Laubach 2008; Ridderinkhof et al. 2004; Swick and Turken 2002), and on dopaminergic signalling therein (Chevrier and Schachar 2010; de Bruijn et al. 2004; Holroyd and Coles 2002; Kattoulas et al. 2010; Kramer et al. 2007). Moreover, these evaluative and regulative functions are found to be impaired in ADHD children (Korenblum et al. 2007; O'Connell et al. 2009; Schachar et al. 2004) and other patient populations.
The present data suggest that DA D3 receptors are involved in the PES component of the SST, consistent with previous literature on the role of DA in error-monitoring and behavioural adjustment (Chevrier and Schachar 2010; de Bruijn et al. 2004; Hester et al. 2012; Holroyd and Coles 2002; Nandam et al. 2012; Nieuwenhuis et al. 2002; Wardle et al. 2012; Zirnheld et al. 2004).
The presence of D3 mRNA in key fronto-striatal loops involved in drug-seeking, relapse to drug addiction and impulsive/compulsive behaviour is consistent with their role in motivation and self-control (Everitt and Robbins 2005; Heidbreder and Newman 2010; Koob and Le Moal 1997). Interestingly, the D3 receptor agonist pramipexole has been associated to the pursuit of risky behaviours (i.e., pathological gambling) in Parkinson’s disease patients receiving this medication (Dodd et al. 2005; Szarfman et al. 2006). However, these effects usually develop only after months of escalating dosage (Dodd et al. 2005), which may explain the inconsistency with the risk-adverse behaviour (St Onge and Floresco 2009) and the increase in post-error behavioural adjustment (present investigation) observed in rats after acute D3 agonist administration. Finally, recent evidence described a significant effect of cabergoline (Nandam et al. 2012), a compound with D3 receptor affinity comparable to that of pramipexole (Gerlach et al. 2003) on error awareness, consistent with the present results.
Summary and conclusions
Converging evidence points to noradrenergic neurotransmission being primarily involved in the therapeutic effects of anti-ADHD drugs (Biederman and Spencer 1999; Robbins and Arnsten 2009). Reduced NA neurotransmission caused by a hypofunctional DA β-hydroxylase — the enzyme responsible for synthesizing NA from DA – produces executive deficits including inattention and impulsivity (Bellgrove et al. 2006; Hess et al. 2009; Kieling et al. 2008). Moreover, the stimulant methylphenidate preferentially releases NA in PFC at clinical doses (Berridge et al. 2006; Kuczenski and Segal 2002), which may underlie its efficacy on ADHD symptoms. Finally, the SNARI atomoxetine improves attention in ADHD patients (Barry et al. 2009; Chamberlain et al. 2007; Maziade et al. 2009), healthy volunteers (Chamberlain et al. 2006a; Marquand et al. 2011) and rodents (Blondeau and Dellu-Hagedorn 2007; Navarra et al. 2008; Robinson et al. 2008). Thus, any attempt to dissect the beneficial effects of generally increasing NA levels in the brain to more specific receptor-mediated modulation of higher cognitive functions, would improve the quality and safety of available pharmacotherapy.
In the present investigation, propranolol administration impaired attentional performance as observed in humans (De Martino et al. 2008; Strange and Dolan 2007). These results suggest that β-adrenergic agonists may be used therapeutically to improve response inhibition and attention, in keeping with the findings that β-adrenoceptor agonists improve response accuracy and impulsivity in the 5-CSRTT (Pattij et al. 2012). Conversely, excessive β-adrenoceptor stimulation as occurs during stress or acute drug withdrawal, may impair cognitive processes (Chamberlain et al. 2006b; Kelley et al. 2005), and β-adrenoceptor blockade is able to reverse this impairment (Alexander et al. 2007; Kelley et al. 2007). Similarly, blockade of α2-adrenoceptors represents a promising target mechanism for future pharmacological treatments of cognitive impairments (Coull et al. 1996; Haapalinna et al. 2000; Sahakian et al. 1994), whereas D3 receptor modulation by pharmacological agents may improve deficits in error monitoring and performance adjustment, which are commonly observed in schizophrenia, ADHD and drug addiction (Carter et al. 2001; Gilmour et al. 2012; Li et al. 2006a; Rubia et al. 2005). Further investigation is warranted to better validate SDGoRT and PES as useful measure, respectively, of sustained attention and dynamic performance adjustment in the rodent SST. Such efforts will contribute to the improvement of the rodent SST as a tool for the screening of drugs directed at ameliorating attention and response control as well as for the investigation of behavioural and cognitive deficits characteristic of ADHD and related disorders.
References
Adams ZW, Roberts WM, Milich R, Fillmore MT (2011) Does response variability predict distractibility among adults with attention-deficit/hyperactivity disorder? Psychol Assess 23:427–436
Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ (2007) Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci 19:468–478
Anden NE, Pauksens K, Svensson K (1982) Selective blockade of brain alpha 2-autoreceptors by yohimbine: effects on motor activity and on turnover of noradrenaline and dopamine. J Neural Transm 55:111–120
Antelman SM, Caggiula AR (1977) Norepinephrine–dopamine interactions and behavior. Science 195:646–653
Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR (1997) Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res 752:26–34
Arnsten AF, Cai JX (1993) Postsynaptic alpha-2 receptor stimulation improves memory in aged monkeys: indirect effects of yohimbine versus direct effects of clonidine. Neurobiol Aging 14:597–603
Arnsten AF, Li BM (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384
Arnsten AF, Cai JX, Goldman-Rakic PS (1988) The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci 8:4287–4298
Arnsten AF, Murphy B, Merchant K (2000) The selective dopamine D4 receptor antagonist, PNU-101387G, prevents stress-induced cognitive deficits in monkeys. Neuropsychopharmacology 23:405–410
Aron AR, Poldrack RA (2005) The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1285–1292
Aston-Jones G, Rajkowski J, Cohen J (2000) Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res 126:165–182
Baldwin RL, Chelonis JJ, Flake RA, Edwards MC, Feild CR, Meaux JB, Paule MG (2004) Effect of methylphenidate on time perception in children with attention-deficit/hyperactivity disorder. Exp Clin Psychopharmacol 12:57–64
Band GP, van der Molen MW, Logan GD (2003) Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 112:105–142
Bari A, Aston-Jones G (2013) Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology 64:53–64
Bari A, Dalley JW, Robbins TW (2008) The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3:759–767
Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW (2009) Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacol (Berl) 205:273–283
Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW (2011) Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci 31:9254–9263
Barik S, de Beaurepaire R (2005) Dopamine D3 modulation of locomotor activity and sleep in the nucleus accumbens and in lobules 9 and 10 of the cerebellum in the rat. Prog Neuropsychopharmacol Biol Psychiatry 29:718–726
Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM (2009) Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology 57:702–707
Bellgrove MA, Mattingley JB, Hawi Z, Mullins C, Kirley A, Gill M, Robertson IH (2006) Impaired temporal resolution of visual attention and dopamine beta hydroxylase genotype in attention-deficit/hyperactivity disorder. Biol Psychiatry 60:1039–1045
Berger B, Tassin JP, Blanc G, Moyne MA, Thierry AM (1974) Histochemical confirmation for dopaminergic innervation of the rat cerebral cortex after destruction of the noradrenergic ascending pathways. Brain Res 81:332–337
Berridge CW, Espana RA (2000) Synergistic sedative effects of noradrenergic alpha(1)- and beta-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience 99:495–505
Berridge CW, Waterhouse BD (2003) The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84
Berridge CW, Arnsten AF, Foote SL (1993) Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models. Psychol Med 23:557–564
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111–1120
Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, Waterhouse BD (2012) Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha(1) - and alpha(2)-receptors. Biol Psychiatry 71:467–473
Bidwell LC, Willcutt EG, Defries JC, Pennington BF (2007) Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiatry 62:991–998
Biederman J, Spencer T (1999) Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry 46:1234–1242
Blondeau C, Dellu-Hagedorn F (2007) Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry 61:1340–1350
Boonstra AM, Kooij JJ, Oosterlaan J, Sergeant JA, Buitelaar JK (2005) Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? J Clin Exp Neuropsychol 27:278–298
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652
Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219
Bowers MB Jr (1984) Homovanillic acid in caudate and pre-frontal cortex following neuroleptics. Eur J Pharmacol 99:103–105
Bradley C (1937) The behavior of children receiving benzedrine. Am J Psychiatry 9:577–585
Brown JW, Braver TS (2005) Learned predictions of error likelihood in the anterior cingulate cortex. Science 307:1118–1121
Brown DC 2nd, Co MS, Wolff RC, Atzori M (2012) alpha-Adrenergic receptors in auditory cue detection: alpha2 receptor blockade suppresses false alarm responding in the rat. Neuropharmacology 62:2178–2183
Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711
Caine SB, Koob GF (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260:1814–1816
Caprioli D, Hong YT, Sawiak SJ, Ferrari V, Williamson DJ, Jupp B, Adrian Carpenter T, Aigbirhio FI, Everitt BJ, Robbins TW, Fryer TD, Dalley JW (2013) Baseline-dependent effects of cocaine pre-exposure on impulsivity and d receptor availability in the rat striatum: possible relevance to the attention-deficit hyperactivity syndrome. Neuropsychopharmacology
Carboni E, Silvagni A (2004) Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol 16:121–128
Carr DB, Andrews GD, Glen WB, Lavin A (2007) alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol 584:437–450
Carter CS, MacDonald AW 3rd, Ross LL, Stenger VA (2001) Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 158:1423–1428
Castellanos FX, Tannock R (2002) Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 3:617–628
Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R (2006) Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci 10:117–123
Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006a) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863
Chamberlain SR, Muller U, Blackwell AD, Robbins TW, Sahakian BJ (2006b) Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacol (Berl) 188:397–407
Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ (2007) Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62:977–984
Chevrier A, Schachar RJ (2010) Error detection in the stop signal task. NeuroImage 53:664–673
Chevrier AD, Noseworthy MD, Schachar R (2007) Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp 28:1347–1358
Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI (1995) The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci 15:1714–1723
Colzato LS, van den Wildenberg WP, Van der Does AJ, Hommel B (2010) Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience 170:782–788
Congdon E, Lesch KP, Canli T (2008) Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet 147B:27–32
Coull JT, Sahakian BJ, Hodges JR (1996) The alpha(2) antagonist idazoxan remediates certain attentional and executive dysfunction in patients with dementia of frontal type. Psychopharmacol (Berl) 123:239–249
Coull JT, Jones ME, Egan TD, Frith CD, Maze M (2004) Attentional effects of noradrenaline vary with arousal level: selective activation of thalamic pulvinar in humans. NeuroImage 22:315–322
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270
Daly SA, Waddington JL (1993) Behavioural effects of the putative D-3 dopamine receptor agonist 7-OH-DPAT in relation to other "D-2-like" agonists. Neuropharmacology 32:509–510
Danielmeier C, Ullsperger M (2011) Post-error adjustments. Front Psychol 2:233
Darracq L, Blanc G, Glowinski J, Tassin JP (1998) Importance of the noradrenaline–dopamine coupling in the locomotor activating effects of d-amphetamine. J Neurosci 18:2729–2739
de Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG (2004) Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacol (Berl) 177:151–160
De Martino B, Strange BA, Dolan RJ (2008) Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacol (Berl) 197:127–136
De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G (1987) Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol 90:675–685
de Wit H, Enggasser JL, Richards JB (2002) Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27:813–825
Depoortere R, Perrault G, Sanger DJ (1996) Behavioural effects in the rat of the putative dopamine D3 receptor agonist 7-OH-DPAT: comparison with quinpirole and apomorphine. Psychopharmacol (Berl) 124:231–240
Depoortere R, Perrault G, Sanger DJ (1999) Intracranial self-stimulation under a progressive-ratio schedule in rats: effects of strength of stimulation, d-amphetamine, 7-OH-DPAT and haloperidol. Psychopharmacol (Berl) 142:221–229
Devauges V, Sara SJ (1990) Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res 39:19–28
Devoto P, Flore G, Pani L, Gessa GL (2001) Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry 6:657–664
Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE (2005) Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol 62:1377–1381
Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP (2002) Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of d-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse 43:51–61
Duarte C, Biala G, Le Bihan C, Hamon M, Thiebot MH (2003a) Respective roles of dopamine D2 and D3 receptors in food-seeking behaviour in rats. Psychopharmacol (Berl) 166:19–32
Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot MH (2003b) Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology 28:1903–1915
Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H (2005) Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry 10:678–685
Eagle DM, Robbins TW (2003) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317
Eagle DM, Tufft MR, Goodchild HL, Robbins TW (2007) Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacol (Berl) 192:193–206
Eagle DM, Bari A, Robbins TW (2008) The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacol (Berl) 199:439–456
Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW (2011) Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci 31:7349–7356
Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, Elliott G, Greenhill LL, Hechtman L, Hoagwood K, Hinshaw SP, Hoza B, Jensen PS, March JS, Newcorn JH, Pelham WE, Severe JB, Swanson JM, Wells K, Vitiello B, Wigal T (2006) Assessing medication effects in the MTA study using neuropsychological outcomes. J Child Psychol Psychiatry 47:446–456
Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, Shiels K, Simon JO, Altaye M (2011) Effects of stimulant medication, incentives, and event rate on reaction time variability in children Wwith ADHD. Neuropsychopharmacology 36(5):1060–1072
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Falkenstein M, Hoormann J, Christ S, Hohnsbein J (2000) ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 51:87–107
Faraone SV, Doyle AE, Mick E, Biederman J (2001) Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry 158:1052–1057
Fernandez-Pastor B, Mateo Y, Gomez-Urquijo S, Javier Meana J (2005) Characterization of noradrenaline release in the locus coeruleus of freely moving awake rats by in vivo microdialysis. Psychopharmacol (Berl) 180:570–579
Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipolito L, Aspinall AT, Robbins TW, Dalley JW (2012) Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacol (Berl) 219:341–352
Fitzpatrick PA, Klorman R, Brumaghim JT, Borgstedt AD (1992) Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. J Am Acad Child Adolesc Psychiatry 31:226–234
Flietstra RJ, Levant B (1998) Comparison of D2 and D3 dopamine receptor affinity of dopaminergic compounds in rat brain. Life Sci 62:1825–1831
Frank MJ, Santamaria A, O'Reilly RC, Willcutt E (2007) Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology 32:1583–1599
Franowicz JS, Arnsten AF (1998) The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacol (Berl) 136:8–14
Gehring WJ, Goss B, Coles MGH, Meyers DE, Donchin E (1993) A neural system for error detection and compensation. Psychol Sci 4:385–390
Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P (2003) Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm 110:1119–1127
Gilmour G, Arguello A, Bari A, Brown VJ, Carter C, Floresco SB, Jentsch DJ, Tait DS, Young JW, Robbins TW (2012) Measuring the construct of executive control in schizophrenia: defining and validating translational animal paradigms for discovery research. Neurosci Biobehav Rev
Gioanni Y, Thierry AM, Glowinski J, Tassin JP (1998) Alpha1-adrenergic, D1, and D2 receptors interactions in the prefrontal cortex: implications for the modality of action of different types of neuroleptics. Synapse 30:362–370
Gobert A, Rivet JM, Cistarelli L, Melon C, Millan MJ (1997) Alpha2-adrenergic receptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine, and serotonin levels in the frontal cortex of freely moving rats. J Neurochem 69:2616–2619
Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160:636–645
Grandoso L, Pineda J, Ugedo L (2004) Comparative study of the effects of desipramine and reboxetine on locus coeruleus neurons in rat brain slices. Neuropharmacology 46:815–823
Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW (2000) Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 20:1208–1215
Gresch PJ, Sved AF, Zigmond MJ, Finlay JM (1995) Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem 65:111–116
Guyon A, Assouly-Besse F, Biala G, Puech AJ, Thiebot MH (1993) Potentiation by low doses of selected neuroleptics of food-induced conditioned place preference in rats. Psychopharmacol (Berl) 110:460–466
Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, Heinonen E (1997) Evaluation of the effects of a specific alpha 2-adrenoceptor antagonist, atipamezole, on alpha 1- and alpha 2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn Schmiedebergs Arch Pharmacol 356:570–582
Haapalinna A, Sirvio J, Lammintausta R (1998) Facilitation of cognitive functions by a specific alpha2-adrenoceptor antagonist, atipamezole. Eur J Pharmacol 347:29–40
Haapalinna A, Sirvio J, MacDonald E, Virtanen R, Heinonen E (2000) The effects of a specific alpha(2)-adrenoceptor antagonist, atipamezole, on cognitive performance and brain neurochemistry in aged Fisher 344 rats. Eur J Pharmacol 387:141–150
Hahn B, Stolerman IP (2005) Modulation of nicotine-induced attentional enhancement in rats by adrenoceptor antagonists. Psychopharmacol (Berl) 177:438–447
Hausknecht KA, Acheson A, Farrar AM, Kieres AK, Shen RY, Richards JB, Sabol KE (2005) Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci 119:302–310
Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, Wyk GW (2011) Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord 15:674–683
Heidbreder CA, Newman AH (2010) Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187:4–34
Helms CM, Gubner NR, Wilhelm CJ, Mitchell SH, Grandy DK (2008) D4 receptor deficiency in mice has limited effects on impulsivity and novelty seeking. Pharmacol Biochem Behav 90:387–393
Hess C, Reif A, Strobel A, Boreatti-Hummer A, Heine M, Lesch KP, Jacob CP (2009) A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J Neural Transm 116:121–130
Hester R, Nandam LS, O'Connell RG, Wagner J, Strudwick M, Nathan PJ, Mattingley JB, Bellgrove MA (2012) Neurochemical enhancement of conscious error awareness. J Neurosci 32:2619–2627
Holmes J, Payton A, Barrett J, Harrington R, McGuffin P, Owen M, Ollier W, Worthington J, Gill M, Kirley A, Hawi Z, Fitzgerald M, Asherson P, Curran S, Mill J, Gould A, Taylor E, Kent L, Craddock N, Thapar A (2002) Association of DRD4 in children with ADHD and comorbid conduct problems. Am J Med Genet 114:150–153
Holroyd CB, Coles MG (2002) The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709
Jakala P, Sirvio J, Riekkinen P Jr, Haapalanna A, Riekkinen P (1992) Effects of atipamezole, an alpha 2-adrenoceptor antagonist, on the performance of rats in a five-choice serial reaction time task. Pharmacol Biochem Behav 42:903–907
Jentsch JD, Taylor JR, Redmond DE Jr, Elsworth JD, Youngren KD, Roth RH (1999) Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacol (Berl) 142:78–84
Jentzsch I, Dudschig C (2009) Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Q J Exp Psychol (Hove) 62:209–218
Ji XH, Ji JZ, Zhang H, Li BM (2008) Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology 33:2263–2271
Jones CR, Malone TJ, Dirnberger G, Edwards M, Jahanshahi M (2008) Basal ganglia, dopamine and temporal processing: performance on three timing tasks on and off medication in Parkinson's disease. Brain Cogn 68:30–41
Kaiser S, Roth A, Rentrop M, Friederich HC, Bender S, Weisbrod M (2008) Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn 66:73–82
Kaneno S, Komatsu H, Fukamauchi F, Ikawa K, Watanabe A (1991) Biochemical basis of antidepressant effect of low dose of sulpiride. Jpn J Psychiatry Neurol 45:131–132
Kaneno S, Fukamauchi F, Komatsu H, Koyama K, Ikawa K (2001) Reversal effect of sulpiride on rotational behaviour of rats with unilateral frontal cortex ablation: an alternative explanation for the pharmacological mechanism of its antidepressant effect. Behav Pharmacol 12:69–73
Karami M, Zarrindast MR (2008) Morphine sex-dependently induced place conditioning in adult Wistar rats. Eur J Pharmacol 582:78–87
Kattoulas E, Evdokimidis I, Stefanis NC, Avramopoulos D, Stefanis CN, Smyrnis N (2010) Monitoring antisaccades: inter-individual differences in cognitive control and the influence of COMT and DRD4 genotype variations. Exp Brain Res 203:453–463
Kelley BJ, Yeager KR, Pepper TH, Beversdorf DQ (2005) Cognitive impairment in acute cocaine withdrawal. Cogn Behav Neurol 18:108–112
Kelley BJ, Yeager KR, Pepper TH, Bornstein RA, Beversdorf DQ (2007) The effect of propranolol on cognitive flexibility and memory in acute cocaine withdrawal. Neurocase 13:320–327
Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (2004) Anterior cingulate conflict monitoring and adjustments in control. Science 303:1023–1026
Khroyan TV, Fuchs RA, Baker DA, Neisewander JL (1997) Effects of D3-preferring agonists 7-OH-PIPAT and PD-128,907 on motor behaviors and place conditioning. Behav Pharmacol 8:65–74
Kieling C, Genro JP, Hutz MH, Rohde LA (2008) The −1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet 147B:485–490
Klein C, Wendling K, Huettner P, Ruder H, Peper M (2006) Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry 60:1088–1097
Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH (2011) Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol 22:300–311
Kohler C, Haglund L, Ogren SO, Angeby T (1981) Regional blockade by neuroleptic drugs of in vivo 3H-spiperone binding in the rat brain. Relation to blockade of apomorphine induced hyperactivity and stereotypies. J Neural Transm 52:163–173
Kollins SH, Anastopoulos AD, Lachiewicz AM, FitzGerald D, Morrissey-Kane E, Garrett ME, Keatts SL, Ashley-Koch AE (2008) SNPs in dopamine D2 receptor gene (DRD2) and norepinephrine transporter gene (NET) are associated with continuous performance task (CPT) phenotypes in ADHD children and their families. Am J Med Genet B Neuropsychiatr Genet 147B:1580–1588
Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58
Korenblum CB, Chen SX, Manassis K, Schachar RJ (2007) Performance monitoring and response inhibition in anxiety disorders with and without comorbid ADHD. Depress Anxiety 24:227–232
Koskinen T, Sirvio J (2001) Studies on the involvement of the dopaminergic system in the 5-HT2 agonist (DOI)-induced premature responding in a five-choice serial reaction time task. Brain Res Bull 54:65–75
Kramer UM, Cunillera T, Camara E, Marco-Pallares J, Cucurell D, Nager W, Bauer P, Schule R, Schols L, Rodriguez-Fornells A, Munte TF (2007) The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J Neurosci 27:14190–14198
Kuczenski R, Segal DS (2002) Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci 22:7264–7271
Lacroix LP, Hows ME, Shah AJ, Hagan JJ, Heidbreder CA (2003) Selective antagonism at dopamine D3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology 28:839–849
LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL (1996) Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry 1:121–124
Lanau F, Zenner MT, Civelli O, Hartman DS (1997) Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. J Neurochem 68:804–812
Langley K, Marshall L, van den Bree M, Thomas H, Owen M, O'Donovan M, Thapar A (2004) Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry 161:133–138
Lapiz MD, Morilak DA (2006) Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137:1039–1049
Lee SH, Shin DW, Stein MA (2010) Increased cortisol after stress is associated with variability in response time in ADHD children. Yonsei Med J 51:206–211
Leth-Steensen C, Elbaz ZK, Douglas VI (2000) Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 104:167–190
Levant B, Vansell NR (1997) In vivo occupancy of D2 dopamine receptors by nafadotride. Neuropsychopharmacology 17:67–71
Li CS, Milivojevic V, Kemp K, Hong K, Sinha R (2006a) Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend 85:205–212
Li J, Wang Y, Zhou R, Zhang H, Yang L, Wang B, Faraone SV (2006b) Association between tryptophan hydroxylase gene polymorphisms and attention deficit hyperactivity disorder in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 141B:126–129
Li CS, Yan P, Chao HH, Sinha R, Paliwal P, Constable RT, Zhang S, Lee TW (2008) Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience 155:1142–1151
Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H (2005) A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol 114:216–222
Lijffijt M, Kenemans JL, ter Wal A, Quik EH, Kemner C, Westenberg H, Verbaten MN, van Engeland H (2006) Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/hyperactivity disorder. Eur Psychiatry 21:544–547
Lindvall O, Bjorklund A (1974) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl 412:1–48
Lipszyc J, Schachar R (2010) Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc 16:1064–1076
Liu YP, Lin YL, Chuang CH, Kao YC, Chang ST, Tung CS (2009) Alpha adrenergic modulation on effects of norepinephrine transporter inhibitor reboxetine in five-choice serial reaction time task. J Biomed Sci 16:72
Logan GD (1994) On the ability to inhibit thought and action. A users' guide to the stop signal paradigm. In: Dagenbach D, Carr TH (eds) Inhibitory processes in attention, memory and language. Academic Press, San Diego, CA, pp 189–236
Loos M, Staal J, Pattij T, Smit AB, Spijker S (2012) Independent genetic loci for sensorimotor gating and attentional performance in BXD recombinant inbred strains. Genes Brain Behav 11:147–156
MacDonald SW, Cervenka S, Farde L, Nyberg L, Backman L (2009) Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia 47:2299–2304
Marquand AF, De Simoni S, O'Daly OG, Williams SC, Mourao-Miranda J, Mehta MA (2011) Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology 36:1237–1247
Matsumoto M, Yoshioka M, Togashi H, Mori K, Ueno K, Saito H (1998) Effects of idazoxan on dopamine release in the prefrontal cortex of freely moving rats. Eur J Pharmacol 343:165–170
Maziade M, Rouleau N, Lee B, Rogers A, Davis L, Dickson R (2009) Atomoxetine and neuropsychological function in children with attention-deficit/hyperactivity disorder: results of a pilot study. J Child Adolesc Psychopharmacol 19:709–718
Mervaala E, Alhainen K, Helkala EL, Partanen J, Jousmaki V, Vayrynen M, Heinonen E, Riekkinen P (1993) Electrophysiological and neuropsychological effects of a central alpha 2-antagonist atipamezole in healthy volunteers. Behav Brain Res 55:85–91
Milstein JA, Dalley JW, Robbins TW (2010) Methylphenidate-induced impulsivity: pharmacological antagonism by beta-adrenoreceptor blockade. J Psychopharmacol 24:309–321
Modirrousta M, Fellows LK (2008) Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci 28:14000–14005
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22:389–395
Muller U, Clark L, Lam ML, Moore RM, Murphy CL, Richmond NK, Sandhu RS, Wilkins IA, Menon DK, Sahakian BJ, Robbins TW (2005) Lack of effects of guanfacine on executive and memory functions in healthy male volunteers. Psychopharmacol (Berl) 182:205–213
Nandam LS, Hester R, Wagner J, Cummins TD, Garner K, Dean AJ, Kim BN, Nathan PJ, Mattingley JB, Bellgrove MA (2010) Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry 69(9):902–904
Nandam LS, Hester R, Wagner J, Dean AJ, Messer C, Honeysett A, Nathan PJ, Bellgrove MA (2012) Dopamine D(2) receptor modulation of human response inhibition and error awareness. J Cogn Neurosci 25(4):649-656
Narayanan NS, Laubach M (2008) Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol 100:520–525
Narayanan NS, Horst NK, Laubach M (2006) Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139:865–876
Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M (2008) Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry 32:34–41
Nayak S, Cassaday HJ (2003) The novel dopamine D4 receptor agonist (PD 168,077 maleate): doses with different effects on locomotor activity are without effect in classical conditioning. Prog Neuropsychopharmacol Biol Psychiatry 27:441–449
Newman-Tancredi A, Audinot-Bouchez V, Gobert A, Millan MJ (1997) Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. Eur J Pharmacol 319:379–383
Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MG, Holroyd CB, Kok A, van der Molen MW (2002) A computational account of altered error processing in older age: dopamine and the error-related negativity. Cogn Affect Behav Neurosci 2:19–36
Oades RD (1987) Attention deficit disorder with hyperactivity (ADDH): the contribution of catecholaminergic activity. Prog Neurobiol 29:365–391
Oak JN, Oldenhof J, Van Tol HH (2000) The dopamine D(4) receptor: one decade of research. Eur J Pharmacol 405:303–327
O'Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, Fitzgerald M, Foxe JJ, Robertson IH (2009) The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD). Neuropsychologia 47:1149–1159
Overtoom CC, Kenemans JL, Verbaten MN, Kemner C, van der Molen MW, van Engeland H, Buitelaar JK, Koelega HS (2002) Inhibition in children with attention-deficit/hyperactivity disorder: a psychophysiological study of the stop task. Biol Psychiatry 51:668–676
Pan WH, Yang SY, Lin SK (2004) Neurochemical interaction between dopaminergic and noradrenergic neurons in the medial prefrontal cortex. Synapse 53:44–52
Passetti F, Levita L, Robbins TW (2003) Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behav Brain Res 138:59–69
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacol (Berl) 191:587–598
Pattij T, Schetters D, Schoffelmeer AN, van Gaalen MM (2012) On the improvement of inhibitory response control and visuospatial attention by indirect and direct adrenoceptor agonists. Psychopharmacol (Berl) 219:327–340
Perry GM, Sagvolden T, Faraone SV (2010a) Intra-individual variability in genetic and environmental models of attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 153B:1094–1101
Perry GM, Sagvolden T, Faraone SV (2010b) Intraindividual variability (IIV) in an animal model of ADHD — the spontaneously hypertensive rat. Behav Brain Funct 6:56
Pertovaara A, Haapalinna A, Sirvio J, Virtanen R (2005) Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev 11:273–288
Pezze MA, Dalley JW, Robbins TW (2007) Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology 32:273–283
Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S (2007) Effects of focal frontal lesions on response inhibition. Cereb Cortex 17:826–838
Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P (1999) Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400:371–375
Puumala T, Riekkinen P Sr, Sirvio J (1997) Modulation of vigilance and behavioral activation by alpha-1 adrenoceptors in the rat. Pharmacol Biochem Behav 56:705–712
Rabbitt PM (1966) Errors and error correction in choice-response tasks. J Exp Psychol 71:264–272
Rabbitt P, Rodgers B (1977) What does a man do after he makes an error? An analysis of response programming. Q J Exp Psychol 29:727–743
Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S (1996) Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav 55:415–422
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306:443–447
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacol (Berl) 163:362–380
Robbins TW, Arnsten AF (2009) The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32:267–287
Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW (2008) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33:1028–1037
Rommelse NN, Altink ME, Oosterlaan J, Beem L, Buschgens CJ, Buitelaar J, Sergeant JA (2008) Speed, variability, and timing of motor output in ADHD: which measures are useful for endophenotypic research? Behav Genet 38:121–132
Rowe JB, Saunders JR, Durantou F, Robbins TW (1996) Systemic idazoxan impairs performance in a non-reversal shift test: implications for the role of the central noradrenergic systems in selective attention. J Psychopharmacol 10:188–194
Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E (2005) Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 162:1067–1075
Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, Sagvolden T (2006) Response variability in attention-deficit/hyperactivity disorder: a neuronal and glial energetics hypothesis. Behav Brain Funct 2:30
Sabol KE, Richards JB, Broom SL, Roach JT, Hausknecht K (2003) Effects of stimulus salience and methamphetamine on choice reaction time in the rat: central tendency versus distribution skew. Behav Pharmacol 14:489–500
Sahakian BJ, Coull JJ, Hodges JR (1994) Selective enhancement of executive function by idazoxan in a patient with dementia of the frontal lobe type. J Neurol Neurosurg Psychiatry 57:120–121
Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A et al (1995) Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J Pharmacol Exp Ther 275:1239–1246
Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AF, Cohen DJ, Leckman JF (2001) A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 158:1067–1074
Scatton B (1977) Differential regional development of tolerance to increase in dopamine turnover upon repeated neuroleptic administration. Eur J Pharmacol 46:363–369
Schachar R, Tannock R, Marriott M, Logan G (1995) Deficient inhibitory control in attention deficit hyperactivity disorder. J Abnorm Child Psychol 23:411–437
Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A (2004) Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J Abnorm Child Psychol 32:285–293
Scheinin H, MacDonald E, Scheinin M (1988) Behavioural and neurochemical effects of antipamezole, a novel alpha 2-adrenoceptor antagonist. Eur J Pharmacol 151:35–42
Selken J, Nichols DE (2007) Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacol Biochem Behav 86:622–630
Sesack SR, Hawrylak VA, Guido MA, Levey AI (1998) Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol 42:171–174
Sibley DR, Strasser RH, Benovic JL, Daniel K, Lefkowitz RJ (1986) Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci U S A 83:9408–9412
Sidhu A, van Oene JC, Dandridge P, Kaiser C, Kebabian JW (1986) [125I]SCH 23982: the ligand of choice for identifying the D-1 dopamine receptor. Eur J Pharmacol 128:213–220
Sirvio J, MacDonald E (1999) Central alpha1-adrenoceptors: their role in the modulation of attention and memory formation. Pharmacol Ther 83:49–65
Sirvio J, Jakala P, Mazurkiewicz M, Haapalinna A, Riekkinen P Jr, Riekkinen PJ (1993) Dose- and parameter-dependent effects of atipamezole, an alpha 2-antagonist, on the performance of rats in a five-choice serial reaction time task. Pharmacol Biochem Behav 45:123–129
Smalley SL, Bailey JN, Palmer CG, Cantwell DP, McGough JJ, Del'Homme MA, Asarnow JR, Woodward JA, Ramsey C, Nelson SF (1998) Evidence that the dopamine D4 receptor is a susceptibility gene in attention deficit hyperactivity disorder. Mol Psychiatry 3:427–430
Smith A, Nutt D (1996) Noradrenaline and attention lapses. Nature 380:291
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151
Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E (2001) The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol 29:215–228
Sorge RE, Clarke PB (2009) Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther 330:633–640
Spencer SV, Hawk LW Jr, Richards JB, Shiels K, Pelham WE Jr, Waxmonsky JG (2009) Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. J Abnorm Child Psychol 37:805–816
St Onge JR, Floresco SB (2009) Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 34:681–697
Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P (2000) Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther 295:1223–1231
Stone EA, Quartermain D (1999) Alpha-1-noradrenergic neurotransmission, corticosterone, and behavioral depression. Biol Psychiatry 46:1287–1300
Strange BA, Dolan RJ (2007) Beta-adrenergic modulation of oddball responses in humans. Behav Brain Funct 3:29
Sun H, Green TA, Theobald DE, Birnbaum SG, Graham DL, Zeeb FD, Nestler EJ, Winstanley CA (2010) Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol Psychiatry 67:649–656
Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG (2005) Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry 57:1209–1211
Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, Moeller FG (2013) Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology (Berl)
Swick D, Turken AU (2002) Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci U S A 99:16354–16359
Szabo ST, Blier P (2001) Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci 13:2077–2087
Szarfman A, Doraiswamy PM, Tonning JM, Levine JG (2006) Association between pathologic gambling and parkinsonian therapy as detected in the Food and Drug Administration Adverse Event database. Arch Neurol 63:299–300, author reply 300
Tanda G, Pontieri FE, Frau R, Di Chiara G (1997) Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci 9:2077–2085
Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD (1989) Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol 17:473–491
Tannock R, Schachar R, Logan G (1995) Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol 23:235–266
Taylor FB, Russo J (2001) Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 21:223–228
Teicher MH, Lowen SB, Polcari A, Foley M, McGreenery CE (2004) Novel strategy for the analysis of CPT data provides new insight into the effects of methylphenidate on attentional states in children with ADHD. J Child Adolesc Psychopharmacol 14:219–232
Thierry AM, Blanc G, Sobel A, Stinus L, Golwinski J (1973) Dopaminergic terminals in the rat cortex. Science 182:499–501
Thierry AM, Le Douarin C, Penit J, Ferron A, Glowinski J (1986) Variation in the ability of neuroleptics to block the inhibitory influence of dopaminergic neurons on the activity of cells in the rat prefrontal cortex. Brain Res Bull 16:155–160
van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ (2006) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacol (Berl) 187:73–85
van Wyk GW, Hazell PL, Kohn MR, Granger RE, Walton RJ (2012) How oppositionality, inattention, and hyperactivity affect response to atomoxetine versus methylphenidate: a pooled meta-analysis. J Atten Disord 16:314–324
Vaurio RG, Simmonds DJ, Mostofsky SH (2009) Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia 47:2389–2396
Verbruggen F, Logan GD (2008) Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12:418–424
Virtanen R (1989) Pharmacological profiles of medetomidine and its antagonist, atipamezole. Acta Vet Scand Suppl 85:29–37
Virtanen R, Savola JM, Saano V (1989) Highly selective and specific antagonism of central and peripheral alpha 2-adrenoceptors by atipamezole. Arch Int Pharmacodyn Ther 297:190–204
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007) Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64:1575–1579
Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22:9595–9603
Wardle MC, Yang A, de Wit H (2012) Effect of d-amphetamine on post-error slowing in healthy volunteers. Psychopharmacol (Berl) 220:109–115
Wilens TE (2008) Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 28:S46–S53
Yamamoto BK, Novotney S (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71:274–280
Young JW, Powell SB, Scott CN, Zhou X, Geyer MA (2011) The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: separating response inhibition from premature responding. Behav Brain Res 222:183–192
Zhang K, Davids E, Tarazi FI, Baldessarini RJ (2002) Effects of dopamine D4 receptor-selective antagonists on motor hyperactivity in rats with neonatal 6-hydroxydopamine lesions. Psychopharmacol (Berl) 161:100–106
Zhang K, Grady CJ, Tsapakis EM, Andersen SL, Tarazi FI, Baldessarini RJ (2004) Regulation of working memory by dopamine D4 receptor in rats. Neuropsychopharmacology 29:1648–1655
Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP (2004) Haloperidol impairs learning and error-related negativity in humans. J Cogn Neurosci 16:1098–1112
Acknowledgements
This work was supported by Wellcome Trust Programme Grant 089589/Z/09/Z awarded to T.W.R., B. J. Everitt,B. J. Sahakian, C. Roberts, and J. W. Dalley and completed within the University of Cambridge Behavioral and Clinical Neuroscience Institute (BCNI), supported by a joint award from the Medical Research Council and Wellcome Trust (Grant G0001354). A.B. was in receipt of a Medical Research Council PhD studentship. The authors thankfully acknowledge D. E. Theobald for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bari, A., Robbins, T.W. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task. Psychopharmacology 230, 89–111 (2013). https://doi.org/10.1007/s00213-013-3141-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3141-6