Abstract
Background
Ketamine, a non-competitive N-methyl-d-aspartic acid antagonist, has been widely used for anaesthetic purposes. At sub-anaesthetic dosage, it induces a dissociative state similar to schizophrenia. The discovery of this effect on dissociative state has led to its use as a pharmacological model of schizophrenia and has also been responsible for its illegal use as a recreational drug. Whereas the former has provided invaluable information, the latter has demonstrated that repeated administration of ketamine induces tolerance. Surprisingly, a review of the relevant literature shows that tolerance to sub-anaesthetic doses of ketamine is largely unreported in neuropharmacological studies.
Methods
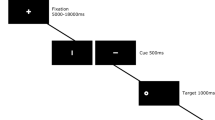
In order to investigate this caveat, we have performed a post hoc analysis of the behavioural effects induced by repeated injections of sub-anaesthetic doses of ketamine observed in five consecutive monkeys performing two oculomotor tasks. Ketamine effects were quantified by the animals' performances and latencies in a prosaccade and an antisaccade task, two oculomotor paradigms that are impaired after ketamine administration.
Results
Although the result of the initial injections confirmed a clear behavioural effect of ketamine injections in all monkeys, subsequent administrations showed that a tolerance eventually appeared in all monkeys. The profile of this tolerance exhibited however a large inter-subject variability.
Conclusions
Psychopharmacological experiments using ketamine as a pharmacological model of psychosis should therefore consider the kinetic and time course of these effects in each individuals and take them into account in the design of experimental protocols.




Similar content being viewed by others
References
Amemori T, Bures J (1990) Ketamine blockade of spreading depression: rapid development of tolerance. Brain Res 519:351–354
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79:565–575
Bree MM, Feller I, Corssen G (1967) Safety and tolerance of repeated anesthesia with CI 581 (Ketamine) in monkeys. Anesth Analg 46:596–600
Bubeníková-Valesová V, Horácek J, Vrajová M, Höschl C (2008) Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev 32:1014–1023
Condy C, Wattiez N, Rivaud-Péchoux S, Gaymard B (2005) Ketamine-induced distractibility: an oculomotor study in monkeys. Biol Psychiatry 57:366–372
Gilmour G, Pioli EY, Dix SL, Smith JW, Conway MW, Jones WT et al (2009) Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology 205:203–216
Godaux E, Cheron G, Mettens P (1990) Ketamine induces failure of the oculomotor neural integrator in the cat. Neurosci Lett 116:162–167
Gunduz-Bruce H (2009) The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev 60:279–286
Kalkman CJ, Drummond JC, Patel PM, Sano T, Chesnut RM (1994) Effects of droperidol, pentobarbital, and ketamine on myogenic transcranial magnetic motor-evoked responses in humans. Neurosurgery 35:1066–1071
Lahti AC, Koffel B, LaPorte D, Tamminga CA (1995) Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13:9–19
Livingston A, Waterman AE (1978) The urinary excretion of ketamine and its metabolites in the rat. Br J Pharmacol 64:402P
Morgan CJ, Huddy V, Lipton M, Curran HV, Joyce EM (2009) Is persistent ketamine use a valid model of the cognitive and oculomotor deficits in schizophrenia? Biol Psychiatry 65:1099–1102
Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJ, Curran HV (2008) Journey through the K-hole: phenomenological aspects of ketamine use. Drug Alcohol Depend 95:219–229
Orser BA, Pennefather PS, MacDonald JF (1997) Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology 86:903–917
Radant AD, Bowdle TA, Cowley DS, Kharasch ED, Roy-Byrne PP (1998) Does ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology 19:434–444
Sinner B, Graf BM (2008) Ketamine, in handbook. Exp Pharmacol 182:313–333
Stefanovic A, Brandner B, Klaassen E, Cregg R, Nagaratnam M, Bromley LM et al (2009) Acute and chronic effects of ketamine on semantic priming: modeling schizophrenia? J Clin Psychopharmacol 29:124–133
Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR (2007) Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull 33:69–94
Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, Iga T (2001) Involvement of CYP2B6 in n-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 29:887–890
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the Institut de Recherche Servier.
Rights and permissions
About this article
Cite this article
Pouget, P., Wattiez, N., Rivaud-Péchoux, S. et al. Rapid development of tolerance to sub-anaesthetic dose of ketamine: an oculomotor study in macaque monkeys. Psychopharmacology 209, 313–318 (2010). https://doi.org/10.1007/s00213-010-1797-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1797-8