Abstract
Rationale
Morphine relieves pain, in part, by acting on neurons within the periaqueductal gray (PAG). Given that the PAG contains a subpopulation of dopamine neurons, dopamine may contribute to the antinociceptive effects mediated by the PAG.
Methods
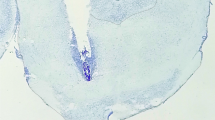
This hypothesis was tested by measuring the behavioral and electrophysiological effects of administering dopamine agonists and antagonists into the ventrolateral PAG (vPAG). An initial histological experiment verified the existence of dopamine neurons within the vPAG using dopamine transporter and tyrosine hydroxylase antibodies visualized with confocal microscopy.
Results
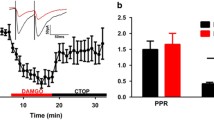
Microinjection of cumulative doses of morphine into the vPAG caused antinociception that was dose-dependently inhibited by the dopamine receptor antagonist α-flupenthixol. α-Flupenthixol had no effect on nociception when administered alone. Injection of the dopamine receptor agonist (−) apomorphine into the vPAG caused a robust antinociception that was inhibited by the D2 antagonist eticlopride but not the D1 antagonist SCH-23390. The effects of dopamine on GABAA-mediated evoked inhibitory post-synaptic potentials (eIPSCs) were measured in PAG slices. Administration of met-enkephalin inhibited peak eIPSCs by 20–50%. Dopamine inhibited eIPSCs by approximately 20–25%. Administration of α-flupenthixol (20 μM) attenuated eIPSC inhibition by dopamine but had no effect on met-enkephalin-induced inhibition.
Conclusions
These data indicate that PAG dopamine has a direct antinociceptive effect in addition to modulating the antinociceptive effect of morphine. The lack of an effect of α-flupenthixol on opioid-inhibition of eIPSCs indicates that this modulation occurs in parallel or subsequent to inhibition of GABA release.






Similar content being viewed by others
References
Bari AA, Pierce RC (2005) D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 135:959–968
Beckstead RM, Domesick VB, Nauta WJ (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175:191–217
Cameron DL, Williams JT (1995) Opposing roles for dopamine and serotonin at presynaptic receptors in the ventral tegmental area. Clin Exp Pharmacol Physiol 22:841–845
Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D (2003) D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci 23:5693–5697
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278
Dong HW, Swanson LW (2006) Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494:142–178
Duttaroy A, Kirtman R, Farrell F, Phillips M, Philippe J, Monderson T, Yoburn BC (1997) The effect of cumulative dosing on the analgesic potency of morphine in mice. Pharmacol Biochem Behav 58:67–71
Flores JA, El Banoua F, Galan-Rodriguez B, Fernandez-Espejo E (2004) Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain 110:205–214
Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, Fernandez-Espejo E (2005) Role for Dopamine Neurons of the Rostral Linear Nucleus and Periaqueductal Gray in the Rewarding and Sensitizing Properties of Heroin. Neuropsychopharmacology 31:1475–1488
Hasue RH, Shammah-Lagnado SJ (2002) Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol 454:15–33
Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ (1998) Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18:10269–10276
Ingram SL, Fossum EN, Morgan MM (2007) Behavioral and Electrophysiological Evidence for Opioid Tolerance in Adolescent Rats. Neuropsychopharmacology 32:600–606
Jensen TS, Smith DF (1982) Role of 5-HT and NA in spinal dopaminergic analgesia. Eur J Pharmacol 86:65–70
Jensen TS, Yaksh TL (1986) Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res 363:99–113
Kalant H, LeBlanc AE, Gibbins RJ (1971) Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev 23:135–191
Kaneto H, Kihara T (1986) Morphine analgesia without development of tolerance in reserpinized mice. Jpn J Pharmacol 42:169–173
Lane DA, Patel PA, Morgan MM (2005) Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience 135:227–234
Lu J, Jhou TC, Saper CB (2006) Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 26:193–202
Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ (2006) Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol 69:1137–1145
Manning BH, Morgan MJ, Franklin KB (1994) Morphine analgesia in the formalin test: evidence for forebrain and midbrain sites of action. Neuroscience 63:289–294
Manzoni OJ, Williams JT (1999) Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci 19:6629–6636
Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2005) Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol 93:3086–3093
Meyer PJ, Fossum EN, Ingram SL, Morgan MM (2007) Analgesic tolerance to microinjection of the μ-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology 52:1580–1585
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225
Morgan MM, Whitney PK, Gold MS (1998) Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain research 804:159–166
Morgan MM, Fossum EN, Levine CS, Ingram SL (2006a) Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav 85:214–219
Morgan MM, Fossum EN, Stalding BM, King MM (2006b) Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain 7:358–366
Nakamura K, Kuntzman R, Maggio A, Conney AH (1973) Restoration of morphine analgesia in morphine-tolerant rats after the intraventricular administration of 6-hydroxydopamine. J Pharm Pharmacol 25:584–587
Nestler EJ (1996) Under siege: The brain on opiates. Neuron 16:897–900
Nicola SM, Malenka RC (1997) Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci 17:5697–5710
Osborne PB, Vaughan CW, Wilson HI, Christie MJ (1996) Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol 490(Pt 2):383–389
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier, Amsterdam
Pert A, Yaksh T (1975) Localization of the antinociceptive action of morphine in primate brain. Pharmacol Biochem Behav 3:133–138
Phillips S, Gelgor L, Mitchell D (1992) Antinociceptive action of dopamine agonists in the nucleus raphe magnus of rats is mediated by D2 receptors. Arch Int Pharmacodyn Ther 319:66–75
Pillai G, Brown NA, McAllister G, Milligan G, Seabrook GR (1998) Human D2 and D4 dopamine receptors couple through betagamma G-protein subunits to inwardly rectifying K+ channels (GIRK1) in a Xenopus oocyte expression system: selective antagonism by L-741,626 and L-745,870 respectively. Neuropharmacology 37:983–987
Reichling DB, Basbaum AI (1990) Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: II. Electron microscopic immunocytochemical evidence of GABAergic control over the projection from the periaqueductal gray to the nucleus raphe magnus in the rat. J Comp Neurol 302:378–393
Rooney KF, Sewell RD (1989) Evaluation of selective actions of dopamine D-1 and D-2 receptor agonists and antagonists on opioid antinociception. Eur J Pharmacol 168:329–336
Seamans JK, Gorelova N, Durstewitz D, Yang CR (2001) Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 21:3628–3638
Self DW (2004) Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 47(Suppl 1):242–255
Simon H, Le Moal M, Calas A (1979) Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Res 178:17–40
Sun W, Rebec GV (2005) The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 177:315–323
Tallarida RJ (2000) Drug synergism and dose effect data analysis. Chapman & Hall, Boca Raton
Tortorici V, Robbins CS, Morgan MM (1999) Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci 113:833–839
Vaughan CW, Christie MJ (1997) Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol 498(Pt 2):463–472
Vaughan CW, Ingram SL, Connor MA, Christie MJ (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390:611–614
Williams JT, Christie MJ, Manzoni O (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343
Wood PL (1983) Opioid regulation of CNS dopaminergic pathways: a review of methodology, receptor types, regional variations and species differences. Peptides 4:595–601
Wood PL, Stotland M, Richard JW, Rackham A (1980) Actions of mu, kappa, sigma, delta and agonist/antagonist opiates on striatal dopaminergic function. J Pharmacol Exp Ther 215:697–703
Yaksh TL, Yeung JC, Rudy TA (1976) Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114:83–103
Acknowledgements
The authors declare that, except for income received from our primary employers, no financial support or compensation has been received from any individual or corporate entity over the for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. This investigation was supported in part by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171, and by NIH grant DA015498.
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was supported, in part, by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171 and by NIH grant DA015498.
Rights and permissions
About this article
Cite this article
Meyer, P.J., Morgan, M.M., Kozell, L.B. et al. Contribution of dopamine receptors to periaqueductal gray-mediated antinociception. Psychopharmacology 204, 531–540 (2009). https://doi.org/10.1007/s00213-009-1482-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1482-y