Abstract
Rationale
We have previously reported that cocaine self-administration is facilitated in male rats not residing in the test chambers (Non Resident rats) relative to rats living in the test chambers at all times (Resident rats). Surprisingly, the opposite was found for heroin.
Materials and methods
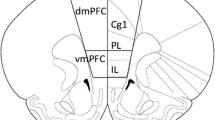
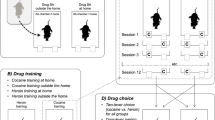
We predicted that, when given access to both cocaine and heroin on alternate days, Non Resident rats would take more cocaine relative to heroin than Resident rats. Heroin (25.0 μg/kg) and cocaine (400 μg/kg), were made alternately available for 14 self-administration sessions, on a fixed ratio (FR) schedule that was progressively increased from FR1 to FR5. Next, some rats underwent a progressive-ratio procedure for heroin and cocaine. The other rats continued to alternate heroin and cocaine self-administration for 12 additional sessions, during which the FR schedule was progressively increased from FR10 to FR100. The second aim of the study was to investigate Fos mRNA expression in Resident and Non Resident rats treated with non-contingent intravenous infusion of “self-administration doses” of heroin (25.0 μg/kg) and cocaine (400 μg/kg).
Results
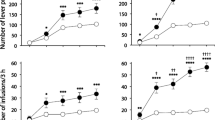
We found that: (1) drug-taking context differentially modulates intravenous cocaine versus heroin self-administration; (2) very low doses of cocaine and heroin are sufficient to induce Fos mRNA expression in the posterior caudate; (3) drug-administration context differentially modulates cocaine- versus heroin-induced Fos mRNA expression.
Conclusions
Our study indicates that the context of drug taking can play a powerful role in modulating cocaine versus heroin intake in the laboratory rat.





Similar content being viewed by others
References
Angerer LM, Cox KH, Angerer RC (1987) Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol 152:649–661
Badiani A, Browman KE, Robinson TE (1995a) Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res 674:291–298
Badiani A, Anagnostaras SG, Robinson TE (1995b) The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 117:443–452
Badiani A, Oates MM, Day HEW, Watson SJ, Akil H, Robinson TE (1998) Amphetamine-induced behavior, dopamine release and c-fos mRNA expression: modulation by environmental novelty. J Neurosci 18:10579–10593
Badiani A, Oates MM, Day HEW, Watson SJ, Akil H, Robinson TE (1999) Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res 103:203–209
Badiani A, Oates MM, Robinson TE (2000a) Modulation of morphine sensitization in the rat by contextual stimuli. Psychopharmacology (Berl) 151:273–282
Cain ME, Smith CM, Bardo MT (2004) The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology (Berl) 176:129–138
Caprioli D, Celentano M, Paolone G, Badiani A (2007a) Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psych 31:1639–1653
Caprioli D, Paolone G, Celentano M, Testa A, Nencini P, Badiani A (2007b) Environmental modulation of cocaine self-administration in the rat. Psychopharmacology (Berl) 192:397–406
Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P, Badiani A (2008) Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology (Berl) 198:395–404
Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A (2009) Ambience and drug choice: cocaine and heroin taking as a function of environmental context in humans and rats. Biol Psychiatry (in press)
Carter BL, Tiffany ST (1999) Meta-analysis of cue-reactivity in addiction research. Addiction 94:327–340
Chang JY, Janak PH, Woodward DJ (1998) Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci 18:3098–3115
Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, McGregor IS (2003) Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol 482:339–341
Crombag HS, Badiani A, Robinson TE (1996) Signalled versus unsignalled intravenous amphetamine: large differences in the acute psychomotor response and sensitization. Brain Res 722:227–231
Crombag H, Bossert JM, Koya E, Shaham Y (2008) Context-induced relapse to drug seeking: a review. Phil Trans R Soc B 363:3233–3243
Curran T, Gordon MB, Rubino KL, Sambucetti LC (1987) Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene 2:79–84
Dalgarno P, Shewan D (1996) Illicit use of ketamine in Scotland. J Psychoactive Drugs 28:191–199
Devine DP, Leone P, Pocock D, Wise RA (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther 266:1236–1246
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278
Dworkin SI, Guerin GF, Co C, Goeders NE, Smith JE (1988) Lack of an effect of 6-hydroxydopamine lesions of the nucleus accumbens on intravenous morphine self-administration. Pharmacol Biochem Behav 30:1051–1057
Ferguson SM, Thomas MJ, Robinson TE (2004) Morphine-induced c-fos mRNA expression in striatofugal circuits: modulation by dose, environmental context, and drug history. Neuropsychopharmacology 29:1664–1674
Gerrits MA, Van Ree JM (1996) Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res 713:114–124
Goeders NE (2003) The impact of stress on addiction. Eur Neuropsychopharmacol 13:435–441
Gysling K, Wang RY (1983) Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 277:119–127
Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ (2006) Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci 24:867–875
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev 56:27–78
Johanson CE, Fischman MW (1989) The pharmacology of cocaine related to its abuse. Pharmacol Rev 41:3–52
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Koob GF (2008) A role for brain stress systems in addiction. Neuron 59:11–34
Kuczenski R, Segal DS (1994) Neurochemistry of amphetamine. In: Cho AK, Segal DS (eds) Amphetamine and its analogs: psychopharmacology, toxicology and abuse. Academic, San Diego, pp 81–113
Matthews RT, German DC (1984) Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience 11:617–625
McElrath K, McEvoy K (2002) Negative experiences on Ecstasy: the role of drug, set and setting. J Psychoactive Drugs 34:199–208
Miczek KA, Covington HE 3rd, Nikulina EM Jr, Hammer RP (2004) Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev 27:787–802
Miller JA (1991) The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB ultrofilm or Amersham hyperfilm. Neurosci Lett 121:211–214
Nace EP (1988) Posttraumatic stress disorder and substance abuse. Clinical issues. Recent Dev Alcohol 6:9–26
Nayak PK, Misra AL, Mulé SJ (1976) Physiological disposition and biotransformation of (3H) cocaine in acutely and chronically treated rats. J Pharmacol Exp Ther 196:556–569
Nestler EJ (2005) Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci 25:210–218
O’Brien CP, Childress AR, Mclellan TA, Ehrman R (1992) Classical conditioning in drug dependent humans. Ann N Y Acad Sci 654:400–415
Paolone G, Burdino R, Badiani A (2003) Dissociation in the modulatory effects of environmental novelty on the locomotor, analgesic, and eating response to acute and repeated morphine in the rat. Psychopharmacology (Berl) 166:146–155
Paolone G, Conversi D, Caprioli D, Del Bianco P, Nencini P, Cabib S et al (2007) Modulatory effect of environmental context and drug history on heroin-induced psychomotor activity and Fos protein expression in the rat brain. Neuropsychopharmacology 32:2611–2623
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Academic, New York
Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 84:167–173
Piazza PV, Le Moal ML (1996) Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36:359–378
Shaham Y, Erb S, Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33:13–33
Singer G, Wallace M (1984) Effects of 6-OHDA lesions in the nucleus accumbens on the acquisition of self injection of heroin under schedule and non schedule conditions in rats. Pharmacol Biochem Behav 20:807–809
Smith JE, Guerin GF, Co C, Barr TS, Lane JD (1985) Effects of 6-OHDA lesions of the central medial nucleus accumbens on rat intravenous morphine self-administration. Pharmacol Biochem Behav 23:843–849
Stallwitz A, Shewan D (2004) A qualitative exploration of the impact of cultural and social factors on heroin use in Shetland (Scotland). J Psychoactive Drugs 36:367–378
Stewart J, de Wit H, Eikelboom R (1984) Role of conditioned and unconditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91:251–268
Strandberg JJ, Kugelberg FC, Alkass K, Gustavsson A, Zahlsen K, Spigset O, Druid H (2006) Toxicological analysis in rats subjected to heroin and morphine overdose. Toxicol Lett 166:11–18
Uslaner J, Badiani A, Norton CS, Day HEW, Watson SJ, Akil H, Robinson TE (2001) Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci 13:1977–1983
Wang H, Gracy KN, Pickel VM (1999) Mu-opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus. J Comp Neurol 412:132–146
Weinshenker D, Schroeder JP (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451
Wikler A (1973) Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch Gen Psychiatry 28:611–616
Wise RA (2004) Dopamine, learning and motivation. Nature Rev Neurosci 5:483–494
Zhang F, Zhou W, Tang S, Lai M, Liu H, Yang G (2004) Motivation of heroin-seeking elicited by drug-associated cues is related to total amount of heroin exposure during self-administration in rats. Pharmacol Biochem Behav 79:291–298
Zinberg NE (1984) Drug, set, and setting the basis for controlled intoxicant use. Yale University Press, New Haven
Acknowledgements
This work was supported by grants from the Sapienza University of Rome (C26A06LHXL and C26F06Y9LL) and from the Italian Ministry for University and Research (PRIN, 2005050334_004). The authors thank Ms. Ilaria Lucchino and Ms. Laura Righetti and for their help with in situ hybridization procedures and data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00213-009-1531-6
Rights and permissions
About this article
Cite this article
Celentano, M., Caprioli, D., Di Pasquale, P. et al. Drug context differently regulates cocaine versus heroin self-administration and cocaine- versus heroin-induced Fos mRNA expression in the rat. Psychopharmacology 204, 349–360 (2009). https://doi.org/10.1007/s00213-009-1467-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1467-x