Abstract
Rationale
Several reports have suggested the involvement of brain adenosine and dopamine receptors in different actions produced by ethanol such as motor incoordination or anxiolytic, hypnotic and reinforcing effects. The co-localization and interaction between adenosine and dopamine receptors in different brain regions has also been well documented. However, few studies have demonstrated the involvement of these mechanisms in the tolerance induced by ethanol.
Objectives
The aim of the present study was to evaluate the role of adenosine and dopamine receptors in the development of rapid tolerance to ethanol-induced motor incoordination in mice.
Methods
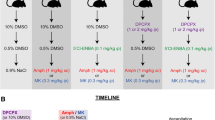
In connection with the rota-rod apparatus, the effects of acute administration of the adenosine receptor antagonists caffeine (non-selective), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, adenosine A1 receptor antagonist) and 4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo-{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM241385, adenosine A2A receptor antagonist), together with R(+)-7-cloro-8-hidroxi-3-metil-1-fenil-2,3,4,5-tetrahidro-1H-3-benzazepine (SCH23390, dopamine D1 receptor antagonist) and sulpiride (dopamine D2 receptor antagonist), alone or in combination with ethanol (2.25 g/kg, i.p.), were studied. Twenty-four hours after, all animals were re-tested on the rota-rod after receiving the same dose of ethanol.
Results
The repeated administration of ethanol promoted a significant reduction of motor impairment on day 2 (i.e. rapid tolerance). This effect was blocked by caffeine (3.0–30.0 mg/kg, i.p.), DPCPX (3.0–6.0 mg/kg, i.p.) or SCH23390 (0.01–0.03 mg/kg, s.c.), but not with ZM241385 (0.5–1.0 mg/kg, i.p.) or sulpiride (1.0–3.0 mg/kg, i.p.).
Conclusions
Our results suggest that the rapid tolerance to ethanol-induced motor impairment in mice may be modulated by adenosine A1 receptors and dopamine D1 receptors.




Similar content being viewed by others
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. (DSM-IV). American Psychiatric Association Publishers, Washington, DC
Appel SB, Liu Z, McElvain MA, Brodie MS (2003) Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther 306:437–446
Barbosa AD, Morato GS (2001) Influence of neurosteroids on the development of rapid tolerance to ethanol in mice. Eur J Pharmacol 431:179–188
Barraco RA, Stefano GB (1990) Pharmacological evidence for the modulation of monoamine release by adenosine in the invertebrate nervous system. J Neurochem 54:2002–2006
Barwick VS, Dar MS (1998) Adenosinergic modulation of ethanol-induced motor incoordination in the rat motor cortex. Prog Neuropsychopharmacol Biol Psychiatry 22:587–607
Bono G, Balducci C, Richelmi P, Koob GF, Pulvirenti L (1996) Dopamine partial receptor agonists reduce ethanol intake in the rat. Eur J Pharmacol 296:233–238
Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO (2004) The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci 7:855–861
Crabbe JC, Rigter H, Uijlen J, Strijbos C (1979) Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther 208:128–133
Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38:107–125
Da Silva GE, Morato GS, Takahashi RN (2001) Rapid tolerance to Δ9-tetrahydro cannabinol and cross-tolerance between ethanol and Δ9-tetrahydrocannabinol in mice. Eur J Pharmacol 431:201–207
Dar MS (1990) Central adenosinergic system involvement in ethanol-induced motor incoordination in mice. J Pharmacol Exp Ther 255:1202–1209
Dar MS (2001) Modulation of ethanol-induced motor incoordination by mouse striatal A1 adenosinergic receptor. Brain Res Bull 55:513–520
Diaz-Cabiale Z, Hurd Y, Guidolin D, Finnman UB, Zoli M, Agnati LF, Vanderhaeghen JJ, Fuxe K, Ferre S (2001) Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum. NeuroReport 12:1831–1834
Drake CL, Roehrs T, Turner L, Scofield HM, Roth T (2003) Caffeine reversal of ethanol effects on the multiple sleep latency test, memory, and psychomotor performance. Neuro-psychopharmacology 28:371–378
El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2003) Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology 45:977–985
Fadda F, Rossetti ZL (1998) Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56:385–431
Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K (1991) Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A 88:7238–7241
Ferre S, Popoli P, Gimenez-Llort L, Finnman UB, Martinez E, Scotti de Carolis A, Fuxe K (1994) Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. NeuroReport 6:73–76
Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K (1997) Adenosine–dopamine receptor–receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20:482–487
Floran B, Barajas C, Floran L, Erlij D, Aceves J (2002) Adenosine A1 receptors control dopamine D1-dependent [(3)H]GABA release in slices of substantia nigra pars reticulata and motor behavior in the rat. Neuroscience 115:743–751
Franks HM, Hagedorn H, Hensley VR, Hensley WJ, Starmer GA (1975) The effect of caffeine on human performance, alone and in combination with ethanol. Psychopharmacology 45:177–181
Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Golembiowska K, Zylewska A (1997) Adenosine receptors: the role in modulation of dopamine and glutamate release in the rat striatum. Pol J Pharmacol 49:317–322
Gonzales RA, Job MO, Doyon WM (2004) The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther 103:121–146
Hunt WA, Majchrowicz E, Dalton TK, Swartzwelder HS, Wixon H (1979) Alterations in neurotransmitter activity after acute and chronic ethanol treatment: studies of transmitter interactions. Alcohol Clin Exp Res 3:359–363
Imperato A, Di Chiara G (1986) Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther 239:219–228
Kalant H (1988) Research on tolerance: what can we learn from history? Alcohol Clin Exp Res 22:67–76
Kebabian JW, Calne DB (1979) Multiple receptors for dopamine. Nature 227:93–96
Khanna JM, Morato GS, Chau A, Shah G, Kalant H (1994) Effect of NMDA antagonists on rapid and chronic tolerance to ethanol: importance of intoxicated practice. Pharmacol Biochem Behav 48:755–763
Khanna JM, Chau A, Shah G (1996) Characterization of the phenomenon of rapid tolerance to ethanol. Alcohol 13:621–628
Khanna JM, Morato GS, Kalant H (2002) Effect of NMDA antagonists, an NMDA agonist, and serotonin depletion on acute tolerance to ethanol. Pharmacol Biochem Behav 72:291–298
Krauss SW, Ghirnikar RB, Diamond I, Gordon AS (1993) Inhibition of adenosine uptake by ethanol is specific for one class of nucleoside transporters. Mol Pharmacol 44:1021–1026
Liguori A, Robinson JH (2001) Caffeine antagonism of alcohol-induced driving impairment. Drug Alcohol Depend 63:123–129
Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS (1990) Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem 265:1946–1951
Ogawa N (1995) Molecular and chemical neuropharmacology of dopamine receptor subtypes. Acta Med Okayama 49:1–11
Okada M, Mizuno K, Kaneko S (1996) Adenosine A1 and A2 receptors modulate extracellular dopamine levels in rat striatum. Neurosci Lett 212:53–56
Popoli P, Gimenez-Llort L, Pezzola A, Reggio R, Martinez E, Fuxe K, Ferre S (1996) Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett 218:209–213
Prediger RDS, Batista LC, Miyoshi E, Takahashi RN (2004a) Facilitation of short-term social memory by ethanol in rats is mediated by dopaminergic receptors. Behav Brain Res 153:149–157
Prediger RDS, Batista LC, Takahashi RN (2004b) Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol 499:147–154
Rossetti ZL, Hmaidan Y, Diana M, Gessa GL (1993) Lack of tolerance to ethanol-induced dopamine release in the rat ventral striatum. Eur J Pharmacol 231:203–207
Schuckit MA (1986) Genetic aspects of alcoholism. Ann Emerg Med 15:991–996
Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR (2002) Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 22:6321–6324
Tabakoff B (1995) Alcohol and tolerance. Alcohol Alert. National Institute on Alcohol Abuse and Alcoholism, vol. 28. NIAAA Press, Bethesda, MD, USA, pp 1–4
Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fuchs S, Janak PH, Gordon AS, Diamond I (2002) betagamma Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell 109:733–743
Yoshimoto K, Ueda S, Nishi M, Yang Y, Matsushita H, Takeuchi Y, Kato B, Kawai Y, Noritake K, Kaneda S, Sorimachi Y, Yasuhara M (2000) Changes in dopaminergic transporter and c-Fos expression in the nucleus accumbens of alcohol-tolerant rats. Alcohol Clin Exp Res 24:361–365
Zaleski MJ, Nunes Filho JR, Lemos T, Morato GS (2001) GABA(B) receptors play a role in the development of tolerance to ethanol in mice. Psychopharmacology 153:415–424
Acknowledgements
The authors are grateful to George E. Silva for his expert comments. L.C.B. and R.D.S.P. are recipients of scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), and G.S.M. and R.N.T. received fellowships from CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batista, L.C., Prediger, R.D.S., Morato, G.S. et al. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology 181, 714–721 (2005). https://doi.org/10.1007/s00213-005-0014-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0014-7