Summary
The subcortical projections to the lateral geniculate body (LGB) in the rat were studied by means of discrete HRP iontophoretic deposits in the dorsal or the ventral LGB; the labelling was compared to that resulting from HRP deposits in neighboring nuclei.
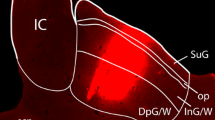
After injecting HRP in the dorsal LGB, labelled cells appeared bilaterally in the ventral LGB, pretectum, superior colliculus, lateral groups of the dorsal raphe nucleus and locus coeruleus. Ipsilaterally, labelled cells were found in the lateral posterior thalamus, nucleus of the posterior commissure and deep mesencephalic reticular nucleus.
After injecting HRP into the ventral LGB, labelled cells were observed bilaterally in the pretectum, superior colliculus and dorsal raphe nucleus (lateral groups). Contralateral labelling appeared in the ventral LGB and parabigeminal nucleus. Ipsilateral labelling was found in the zona incerta, lateral posterior thalamus, lateral and medial mesencephalic reticular formation, vestibular and dorsal tegmental nuclei.
These findings provide evidence of subcortical projections to the LGB arising in visually-related areas as well as extravisual areas, which might be related to the LGB boutons that survive complete cortical and retinal ablations.
Similar content being viewed by others
References
Albano JE, Norton TT, Hall WC (1979) Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Res 173: 1–11
Altman J, Bayer SA (1979) Development of the diencephalon in the rat. V. Thymidine-radiographic observations on internuclear and intranuclear gradients in the thalamus. J Comp Neurol 188: 473–500
Altman J, Carpenter MB (1961) Fiber projections of the superior colliculus in the cat. J Comp Neurol 116: 157–177
Angel A, Magni F, Strata P (1965) Excitability of intra-geniculate optic tract fibers after reticular stimulation in the mid-pontine pretrigeminal cat. Arch Ital Biol 103: 668–693
Angevine JB (1970) Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol 139: 129–188
Berman N (1977) Connections of the pretectum in the cat. J Comp Neurol 174: 227–254
Berson DM, Graybiel AM (1978) Parallel thalamic zones in the LP-pulvinar complex of the cat identified by either afferent and efferent connections. Brain Res 147: 139–148
Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M (1976) The raphe nuclei of the cat brain stem: A topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113: 449–486
Bowsher D (1970) Reticular projections to lateral geniculate in cat. Brain Res 23: 247–249
Burke W, Sefton AJ (1966) Inhibitory mechanisms in lateral geniculate nucleus of rat. J Physiol (Lond) 187: 231–246
Buser P, Segundo J (1959) Influences réticulaires somesthésiques et corticales au niveau du corps genouillé latéral du thalamus chez le chat. CR Seances Acad Sci (Paris) 249: 571–573
Conrad LCA, Leonard CM, Pfaff DW (1974) Connections of the median and dorsal raphe nuclei in the rat: An autoradiographic and degeneration study. J Comp Neurol 156: 179–206
Cullen MJ, Kaiserman-Abramof IR (1976) Cytological organization of the dorsal lateral geniculate nuclei in mutant anophthalmic and postnatally enucleated mice. J Neurocytol 5: 407–424
Fukuda Y, Iwama K (1971) Reticular inhibition of internuncial cells in the rat lateral geniculate body. Brain 35: 107–118
Fuster JM, Creutzfeldt OD, Straschill M (1965) Intracellular recording of neuronal activity in the visual system. Z Vgl Physiol 49: 605–622
Garey LJ, Jones EG, Powell TPS (1968) Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway. J Neurol Neurosurg Psychiatry 31: 135–157
Gobel S, Falls WM, Humphrey E (1981) Morphology and synaptic connections of ultrafine primary axons in lamina I of the spinal dorsal horn: Candidates for terminal axonal arbors of primary neurons with unmyelinated (c) axons. J Neurosci 1: 1163–1179
Graham S (1977) An autoradiographic study of the efferent connections of the superior colliculus in the cat. J Comp Neurol 173: 629–654
Graybiel AM (1972) Some extrageniculate visual pathways in the cat. Invest Ophthalmol Vis Sci 11: 322–332
Graybiel AM (1974) Visuo-cerebellar and cerebello-visual connections involving the ventral lateral geniculate nucleus. Exp Brain Res 20: 303–306
Graybiel AM (1978) A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res 145: 365–374
Gurdjian ES (1927) The diencephalon of the albino rat. Studies on the brain of the rat. J Comp Neurol 43: 1–114
Hanker JS, Yates PE, Metz CB, Rustioni A (1977) A new specific sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J 9: 789–792
Hickey TL, Guillery RW (1974) An autoradiographic study of retinogeniculate pathways in the cat and the fox. J Comp Neurol 156: 239–254
Holcombe V, Hall WC (1981) The laminar origin and distribution of the crossed tectoreticular pathways. J Neurosci 1: 1103–1112
Hughes HC (1980) Efferent organization of the cat pulvinar complex, with a note on bilateral claustrocortical and reticulocortical connections. J Comp Neurol 193: 937–963
Hughes CP, Chi Dyk (1981) Afferent projections to the ventral lateral geniculate nucleus in the cat. Brain Res 207: 445–448
Itoh K, Mizuno N, Sugimoto T, Nomura S, Nakamura Y, Konishi A (1979) A cerebello-pulvino-cortical and a retino-pulvinocortical pathways in the cat as revealed by the use of anterograde and retrograde transport of horseradish peroxidase. J Comp Neurol 187: 349–358
Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127: 23–53
Jordan H, Hollander H (1972) The structure of the ventral part of the lateral geniculate nucleus. A cyto- and myeloarchitectonic study in the cat. J Comp Neurol 145: 259–272
Kaas JH, Guillery RW, Allman JM (1972) Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol 6: 253–299
Kawamura S, Fukushima N, Hattori S (1979) Topographical origin and ganglion cell type of the retino-pulvinar projection in the cat. Brain Res 173: 419–429
Konig JFR, Klippel RA (1967) The rat brain. A stereotaxic atlas of the forebrain and lower part of the brain stem. Krieger, Huntington
Kriebel RM (1975) Neurons of the dorsolateral geniculate nucleus of the albino rat. J Comp Neurol 159: 45–68
Kromer LF, Moore RY (1980) A study of the organization of the locus coeruleus projections to the lateral geniculate nucleus in the albino rat. Neuroscience 5: 255–271
Kuypers HGJM, Lawrence DG (1967) Cortical projections to the red nucleus and the brain stem in the rhesus monkey. Brain Res 4: 151–158
Leger L, Sakai K, Salvert D, Touret M, Jouvet M (1975) Delineation of the dorsal lateral geniculate afferents from the cat brain stem as visualized by the horseradish peroxidase technique. Brain Res 93: 490–496
Legg CR (1979) Visual discrimination impairments after lesions in zona incerta or lateral terminal nucleus of accessory optic tract. Brain Res 177: 461–478
Lieberman AR, Webster KE (1974) Aspects of the synaptic organization of intrinsic neurons in the dorsal lateral geniculate nucleus. An ultrastructural study of the normal and of the experimentally deafferented nucleus in the rat. J Neurocytol 3: 677–710
Maeda T, Shimizu N (1972) Projections ascendantes du locus coeruleus et d'autres neurones aminergiques pontiques au niveau du prosencéphale du rat. Brain Res 36: 19–35
Magnin M, Kennedy H (1979) Anatomical evidence of a third ascending vestibular pathway involving the ventral lateral geniculate nucleus and the intralaminar nuclei of the cat. Brain Res 171: 523–529
Meikle TH, Sprague JM (1964) The neural organization of the visual pathways in the cat. Int Rev Neurobiol 6: 149–189
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neuro-histochemistry: A non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Moore RY, Halaris AE, Jones BE (1978) Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol 180: 417–438
Nauta WJH, Bucher VM (1954) Efferent connections of the striate cortex in the albino rat. J Comp Neurol 100: 257–285
Palkovits M, Jacobowitz DM (1974) Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (Mesencephalon, Rhombencephalon). J Comp Neurol 157: 29–42
Pasquier DA, Reinoso-Suárez F (1977) Differential efferent connections of the brain stem to the hippocampus in the cat. Brain Res 120: 540–548
Pasquier DA, Reinoso-Suárez F (1978) The topographic organization of hypothalamic and brain stem projections to the hippocampus. Brain Res Bull 3: 373–389
Pasquier DA, Tramezzani JH (1979) Afferent connections of the hypothalamic retrochiasmatic area in the rat. Brain Res Bull 4: 765–771
Polyak S (1957) The vertebrate visual system. University of Chicago Press, Chicago
Ramón y Cajal S (1904) Textura del sistema nervioso del hombre y de los vertebrados, vol II. Madrid, Spain
Ribak EC, Peters A (1975) An autoradiographic study of the projections from the lateral geniculate body of the rat. Brain Res 92: 341–368
Ricardo JA (1981) Efferent connections of the subthalamic region in the rat. II. The zona incerta. Brain Res 214: 43–60
Robson JA, Hall WC (1976) Projections from the superior colliculus to the dorsal lateral geniculate nucleus of the grey squirrel (Sciurus carolinensis). Brain Res 113: 379–385
Robson JA, Hall WC (1977) The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). I. Cytoarchitecture and connections. J Comp Neurol 173: 355–388
Scheibel ME, Scheibel AB (1966) Patterns of organization in specific and nonspecific thalamic fields. In: Purpura D, Yahr MD (eds) The thalamus. Columbia University Press, New York, pp 13–46
Swanson LW, Cowan WM, Jones EG (1974) An autoradiographic study of th efferent connections of the ventral lateral geniculate nucleus in the albino rat and the cat. J Comp Neurol 156: 143–164
Swanson LW, Hartman BK (1975) The central adrenergic system. An immuno-fluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-Bhydroxylase as a marker. J Comp Neurol 163: 467–506
Szentágothai J (1973) Neuronal and synaptic architecture of the lateral geniculate nucleus. In: Jung R (ed) Central processing of visual information. Part B, Morphology and function of visual centers in the brain. Springer, Berlin Heidelberg New York (Handbook of sensory physiology, vol VII-3, pp 141–176)
Vay S Le (1971) On the neurons and synapses of the lateral geniculate nucleus of the monkey, and the effect of eye enucleation. Z Zellforsch 113: 396–419
Villar MJ, Huerta M, Pasquier DA (1979) Dorsal raphe nucleus projecting to the superior colliculus and the lateral geniculate nucleus in the rat. Soc Neurosci Abstr 5: 355
Watanabe K, Kawana E (1979) Efferent projections of the parabigeminal nucleus in rats: A horseradish peroxidase (HRP) study. Brain Res 168: 1–11
Wilson PD, Pecci-Saavedra J, Doty RW (1973) Mesencephalic control of lateral geniculate nucleus in primates. II. Effective loci. Exp Brain Res 18: 204–213
Wyss JM, Swanson LW, Cowan WM (1979) A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience 4: 463–476
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pasquier, D.A., Villar, M.J. Subcortical projections to the lateral geniculate body in the rat. Exp Brain Res 48, 409–419 (1982). https://doi.org/10.1007/BF00238617
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00238617