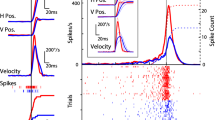
Summary
-
1.
Chronically prepared, awake cats were rotated in the horizontal plane with the head fixed. Single unit activity from the lateral geniculate body (LGB) was recorded together with eye position.
-
2.
Over 165 neurons, 101 (61%) were influenced by rotation. 64 detected only the onset and the offset of vestibular stimulation without respect to the direction or the velocity of the rotatory movement. Global increase in firing was by far the most frequent response. A large proportion of these neurons were located within the dorsal nucleus of LGB, and had discrete receptive fields.
-
3.
30 other neurons were influenced by rotation asymetrically. In 22 cases, firing rate increased during rotation contralateral to the recorded side. The reverse pattern was observed in 8 cases. In the direction of rotation where firing increased, maximum firing rate usually corresponded to the maximum amplitude of the velocity vector. In the opposite direction, firing decreased only slightly with respect to background activity.
-
4.
The 7 other neurons were influenced only by angular velocity, and not by direction of rotation. Firing increased during rotation, and stopped when the direction was reverted, i.e., when velocity was equal to zero.
-
5.
Neurons described under (3) and (4) poorly responded to light. Only 4 had a discrete receptive field. A large proportion were located in the ventral nucleus of LGB.
-
6.
Over 157 neurons from the same sample, 82 (52%) were influenced by saccades in the dark. This number includes different types of saccade-locked changes. The most frequent type was an increase in firing about 250 msec after saccade onset, and clearly related to saccades in one direction (e.g., right or left) only. About a half of the neurons influenced by saccades responded to light, and 21 had a discrete receptive field. A large proportion of neurons influenced by saccades were located within the dorsal nucleus of LGB. However, about 20 were located in the ventral nucleus; they correspond to neurons described under (3), which also responded to the direction of rotation.
-
7.
Possible contributions of these vestibular and saccadic influences on LGB to the control of visuomotor behavior, are discussed.
Similar content being viewed by others
References
Altman, J., Carpenter, M.B.: Fiber projections of the superior colliculus in the cat. J. comp. Neurol. 116, 157–178 (1961)
Angaut, P.: The fastigiotectal projection. An anatomical experimental study. Brain Res. 13, 186–189 (1969)
Berlucchi, G., Munson, J.B., Rizzolatti, G.: Surgical immobilization of the eye and pupil, permitting stable photic stimulation of freely moving cats. Electroenceph. clin. Neurophysiol. 21, 504–505 (1966)
Bisti, S., Maffei, L., Piccolino, M.: Variations of the visual responses of the superior colliculus in relation to body roll. Science 175, 456–457 (1972)
Bizzi, E.: Vestibular effects on the visual input. In: Handbook of Sensory Physiology, the vestibular system. Ed. by H.H. Kornhuber. Berlin-Heidelberg-New York: Springer (in press)
Bond, H.W., Ho, P.: Solid miniature silver-silver chloride electrodes for chronic implantation. Electroenceph. clin. Neurophysiol. 28, 206–208 (1970)
Bowsher, D.: Reticular projections to lateral geniculate in cat. Brain Res. 23, 247–249 (1970)
Brodal, A.: Anatomy of the vestibulo reticular connections and possible “ascending” vestibular pathways from the reticular formation. Progr. Brain Res. 37, 553–565 (1972)
Buser, P., Segundo, J.P.: Influences reticulaires somesthésiques et corticales au niveau du corps genouillé latéral du thalamus chez le chat. C.R. Acad. Sci. (Paris) 249, 571–573 (1959)
Buttner, U., Fuchs, A.F.: Influence of saccadic eye movements on unit activity in simian lateral geniculate and pregeniculate nuclei. J. Neurophysiol. 36, 127–141 (1973)
Campbell, C.B.G.: Evolutionary patterns in mammalian diencephalic visual nuclei and their fiber connections. Brain Behav. Evol. 6, 218–236 (1972)
Corrazza, R., Lombroso, C.T.: The neuronal dark discharge during eye movements in the cat. Brain Res. 34, 345–360 (1971)
Cragg, B.G.: The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 9, 733–747 (1969)
Duensing, F., Schaefer, K.P.: Die Aktivität einzelner Neurone im Bereich der Vestibulariskerne bei Horizontalbeschleunigungen unter besonderer Berücksichtigung des vestibulären Nystagmus. Arch. Psychiat. Nervenkr. 198, 225–252 (1958)
Gernandt, B.: Response of mammalian vestibular neurons to horizontal rotation and caloric stimulation. J. Neurophysiol. 12, 173–184 (1949)
Gernandt, B.: Midbrain activity in response to vestibular stimulation. Acta physiol. scand. 21, 73–81 (1950)
Gernandt, B.E.: Vestibular mechanisms. In: J. Field, H.W. Magoun and V.E. Hall (Eds.). Handbook of Physiology, Neurophysiology, Vol. I, pp. 549–564. Washington: American Physiological Society 1959
Glickstein, M., King, R.A., Miller, J., Berkley, M.: Cortical projections from the dorsal lateral geniculate nucleus of cats. J. comp. Neurol. 130, 55–76 (1967)
Goldberg, J.M., Fernández, C.: Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J. Neurophysiol. 34, 635–660 (1971)
Graybiel, A.M.: Visuo-cerebellar and cerebello-visual connections involving the ventral lateral geniculate nucleus, Exper. Brain Res. 20, 303–306 (1974)
Gregory, R.L.: Eye movements and the stability of the visual world. Nature (Lond.) 182, 1214–1216 (1958)
Grusser, O.J., Grusser-Cornhels, U., Saur, G.: Reaktionen einzelner Neurone im optischen Cortex der Katze nach elektrischer Polarisation des Labyrinths. Pflügers Arch. ges. Physiol. 259, 593–612 (1959)
Hollander, H.: The projection from the visual cortex to the lateral geniculate body. An experimental study with silver impregnation methods in the cat. Exp. Brain Res. 10, 219–235 (1970)
Holst, E. von: Relations between the central nervous system and the peripheral organs. Brit. J. Anim. Behav. 2, 89–94 (1954)
Jasper, H.H., Ajmone-Marsan, C.: A stereotaxic atlas of the diencephalon of the cat. National Research Council of Canada. Ottawa, 1954
Jeannerod, M.: Saccade-correlated events in the lateral geniculate body. Bibl. ophthal. (Basel) 82, 189–198 (1972)
Jeannerod, M., Putkonen, P.T.S.: Oculomotor influences on lateral geniculate body neurons. Brain Res. 24, 125–129 (1970)
Jeannerod, M., Putkonen, P.T.S.: Lateral geniculate unit activity and eye movements: saccade-locked changes in dark and in light. Exp. Brain Res. 13, 533–546 (1971)
Jones, G.M., Milsum, J.H.: Characteristics of neural transmission from the semicircular canal to the vestibular nuclei of cats. J. Physiol. (Lond.) 209, 295–316 (1970)
Jordan, H., Hollander, H.: The structure of the ventral part of the lateral geniculate nucleus. A cyto and myelo-architectonic study in the cat. J. comp. Neurol. 145, 259–272 (1972)
Jung, R., Kornhuber, H.H., Da Fonseca, J.S.: Multisensory convergence on cortical neurons. In: Brain mechanisms, Moruzzi, Fessard and Jasper, Editors. Amsterdam: Elsevier 1963
Kornhuber, H.H., Da Fonseca, J.J.: Optovestibular integration in the cat's cortex. A study of sensory convergence on cortical neurons. In: M.B. Bender, Ed.: The oculomotor system, pp. 239–279. New York: Harper and Row 1964
Laties, A.M., Sprague, J.M.: The projection of optic fibers to the visual centers of the cat. J. comp. Neurol. 127, 35–70 (1966)
Magnin, M., Jeannerod, M.: Fixation non traumatique de la tête chez le chat éveillé. C.R. Soc. Biol. (Paris) 167, 996–998 (1973)
Marty, R., Benoit, O., Larguier, M.M.: Etude topographique et stratigraphique des projections du corps genouillé latéral sur le cortex cérébral. Arch. ital. Biol. 107, 723–742 (1969)
Melvill Jones, G., Milsum, J.H.: Frequency response analysis of central vestibular unit activity resulting from rotational stimulation of the semicircular canals. J. Physiol. (Lond.) 219, 191–215 (1971)
Meulders, M., Colle, J., Godfraind, J.M.: Evoked sensory responses of somatic origin in the lateral geniculate body. Arch. int. Physiol. Biochem. 72, 346 (1964)
Mickle, W.A., Ades, H.W.: Rostral projection pattern of the vestibular system. Amer. J. Physiol. 176, 243–246 (1954)
Montero, V.M., Robles, L.: Saccadic modulation of cell discharges in the lateral geniculate nucleus. Vision Res. Suppl. 3, 253–268 (1971)
Morrell, F.: Integrative properties of parastriate neurons. In: Brain and Human behavior, Karczmar and Eccles, Ed. pp. 259–289. Berlin-Heidelberg-New York: Springer 1972
Mukhametov, L.M., Rizzolati, G., Trabardi, V.: Spontaneous activity of neurons of nucleus reticularis thalami in freely moving cats. J. Physiol. (Lond.) 210, 651–667 (1970)
Nauta, W.J.H., Kuypers, H.G.J.M.: Some ascending pathways in the brainstem reticular formation. In: H. Jasper, Ed.: Reticular formation of the brain, pp. 3–30. Boston: Little, Brown & Co. 1958
Niimi, K., Sprague, J.M.: Thalamo cortical organisation of the visual system in the cat. J. comp. Neurol. 138, 219–250 (1970)
Niimi, K., Kanaseki, T., Takimoto, T.: The comparative anatomy of the ventral nucleus of the lateral geniculate body in mammals. J. comp. Neurol. 121, 313–324 (1963)
Noda, H., Freeman, R.B., Creutzfeldt, O.D.: Neuronal correlates of eye movements in the visual cortex of the cat. Science 175, 661–664 (1972)
Ogawa, T.: Midbrain reticular influences upon single neurons in lateral geniculate nucleus. Science 139, 343–344 (1963)
Papaioannou, J.: Electrical stimulation of vestibular nuclei: effects on spontaneous activity of lateral geniculate nucleus neurons. Arch. ital. Biol. 110, 217–233 (1972a)
Papaioannou, J.: Electrical stimulation of vestibular nuclei: effects on light evoked activity of lateral geniculate nucleus neurons. Pflügers Arch. 334, 129–140 (1972b)
Papaioannou, J.: Effects of caloric labyrinthine stimulation on the spontaneous activity of lateral geniculate nucleus neurons in the cat. Exp. Brain Res. 17, 1–9 (1973)
Pernier, J., Echallier, J.F., Gerin, P.: Système d'analyse des relations entre phénomènes lents et activité neuronale (In preparation)
Polyak, S.: The vertebrate visual system. Chicago: University of Chicago Press 1957
Putkonen, P.T.S., Magnin, M., Jeannerod, M.: Directional responses to head rotation in neurons, from the ventral nucleus of the lateral geniculate body. Brain Res. 61, 407–411 (1973)
Roucoux, A., Crommelink, M.: Influence of nystagmic and spontaneous activity on superior colliculus neurons in curarized cats. Experientia (Basel) 27, 1181–1182 (1971)
Roucoux, A., Crommelink, M., Roucoux-Hanus, M.: Potentiels évoqués au niveau des tubercules quadrijumeaux antérieurs du chat par la stimulation labyrinthique. J. Physiol. (Paris) 65, 488 A (1972)
Satinsky, D.: Reticular influences on lateral geniculate neurone activity. Electroenceph. clin. Neurophysiol. 25, 543–549 (1968)
Scheibel, M.E., Scheibel, A.B.: Structural substrates for integrative patterns in the brainstem reticular core. In: H. Jasper, Ed.: Reticular formation of the brain, pp. 31–55. Boston: Little, Brown & Co. 1958
Scheibel, M.E., Scheibel, A.B.: The organization of the nucleus reticularis thalami: a golgi study. Brain Res. 1, 43–62 (1966)
Schwartzkroin, P.A.: Effects of round window stimulation on unit discharges in the visual cortex and superior colliculus. Exp. Brain Res. 17, 527–538 (1973)
Shimazu, H., Precht, W.: Tonic and kinetic responses of cat's vestibular neurons to horizontal angular accelerations. J. Neurophysiol. 28, 991–1013 (1965)
Singer, W.: The effect of mesencephalic reticular stimulation on intracellular potentials of cat lateral geniculate neurons. Brain Res. 61, 35–54 (1973)
Singer, W., Dräger, U.: Postsynaptic potential in relay neurons of cat lateral geniculate nucleus after stimulation of the mesencephalic reticular formation. Brain Res. 41, 214–220 (1972)
Szentágothai, J.: Lateral geniculate body structure and eye movement. Bibl. ophthal. (Basel) 82, 178–188 (1972)
Tatton, W.G., Crapper, D.R.: Central tegmental alteration of oat lateral geniculate activity. Brain Res. 47, 371–387 (1972)
Wepsic, J.G.: Multimodal sensory activation of cells in the magnocellular medial geniculate nucleus. Exp. Neurol. 15, 299–318 (1966)
Wilson, M.E., Cragg, B.G.: Projections from the lateral geniculate nucleus in the cat and the monkey. J. Anat. (Lond.) 101, 677–692 (1967)
Wurtz, R.H.: Comparison of effects of eye movements and stimulus movements on striate cortex neurons of the monkey. J. Neurophysiol. 32, 987–994 (1969)
Author information
Authors and Affiliations
Additional information
Supported by INSERM and FRMF, Paris. We wish to thank also U.E.R. de Biologie Humaine, Université Claude Bernard, Lyon, for providing a grant to M.M.
Rights and permissions
About this article
Cite this article
Magnin, M., Jeannerod, M. & Putkonen, P. Vestibular and saccadic influences on dorsal and ventral nuclei of the lateral geniculate body. Exp Brain Res 21, 1–18 (1974). https://doi.org/10.1007/BF00234255
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00234255