Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome
- 1Laboratory of Neurobiology, Scuola Normale Superiore, Pisa, Italy
- 2Department of Psychology, Florence University, Florence, Italy
- 3Institute of Neuroscience CNR, Pisa, Italy
- 4Department of Experimental Medicine, Section of Pharmacology and Toxicology, University of Genova, Genova, Italy
- 5Department of Developmental Neuroscience, IRCCS Stella Maris and University of Pisa, Calambrone, Pisa, Italy
Down syndrome (DS) is the most common genetic disorder associated with mental retardation. It has been repeatedly shown that Ts65Dn mice, the prime animal model for DS, have severe cognitive and neural plasticity defects due to excessive inhibition. We report that increasing sensory-motor stimulation in adulthood through environmental enrichment (EE) reduces brain inhibition levels and promotes recovery of spatial memory abilities, hippocampal synaptic plasticity, and visual functions in adult Ts65Dn mice.
Introduction
Down syndrome (DS), a condition due to chromosome 21 trisomy (Lejeune et al., 1959), is the most common genetic cause of mental retardation, with an incidence ranging from 1 in 700 to 1 in 1000 live births (Roizen and Patterson, 2003). Together with cognitive disabilities (Nadel, 2003; Pennington et al., 2003), people with DS exhibit a number of attention and sensory deficits (Brown et al., 2003; Clark and Wilson, 2003), with well-documented defects in basic visual functions (John et al., 2004). In search for possible molecular and cellular processes involved in the pathogenesis of the syndrome, several transgenic mouse models of DS have been generated, carrying triplications of different segments of the murine chromosome 16, which has a large degree of synteny with the human chromosome 21 (Gardiner et al., 2003; Seregaza et al., 2006; Moore and Roper, 2007). The most widely studied model of DS is the Ts65Dn line, which summarizes the main hallmarks of the DS phenotype, including a serious cognitive impairment (Escorihuela et al., 1995; Reeves et al., 1995; Holtzman et al., 1996; Hunter et al., 2003; Stasko and Costa, 2004) and multiple deficits at the functional and anatomical level in the visual system (Scott-McKean et al., 2010; Blank et al., 2011).
Numerous studies have shown that the cognitive deficits displayed by Ts65Dn mice are mainly related to excessive levels of inhibition in the temporal lobe circuitries, with a failure of long-term synaptic plasticity in the hippocampus (e.g., Siarey et al., 1997, 1999; Kleschevnikov et al., 2004, 2011; Fernandez et al., 2007; see Baroncelli et al., 2011 for a recent review). Accordingly, marked morphological changes in inhibitory circuitries have been reported in the hippocampus and in the cerebral cortex, with a selective enlargement of the active zones of symmetric synapses and increased immunoreactivity for synaptic proteins marking inhibitory synapses (Belichenko et al., 2009; Perez-Cremades et al., 2010). Moreover, systemic administration of non-competitive antagonists of GABAA receptors can reverse spatial learning disabilities and long-term potentiation (LTP) deficits in Ts65Dn mice (Fernandez et al., 2007).
Despite this evidence, however, it remains unknown whether it is possible to ameliorate the phenotype of DS mouse models using non-invasive approaches more eligible for human application. Evidence exists that environmental enrichment (EE), a condition of increased sensory-motor stimulation (van Praag et al., 2000; Sale et al., 2009), has remarkably beneficial effects in animal models of nervous system disorders, including neurodegenerative and developmental diseases (for comprehensive reviews, see Nithianantharajah and Hannan, 2006; Baroncelli et al., 2010a). It has been demonstrated that exposing Ts65Dn mice to EE improves their memory abilities (Martínez-Cué et al., 2002, 2005), affects the structure of cortical pyramidal cells (Dierssen et al., 2003) and, at the molecular level, restores the G-protein-associated signal transduction systems (Baamonde et al., 2011). Moreover, a combination of EE and physical exercise increases cell proliferation, neurogenesis, and gliogenesis in the hippocampus and the forebrain subventricular zone (Chakrabarti et al., 2011). Very little is instead known on the EE impact at the level of neural functions in the hippocampus and cerebral cortex of DS mouse models.
Using the visual system as a paradigm for the study of experience-dependent plasticity, we have recently demonstrated that EE can be efficiently employed to reduce cortical inhibition (Sale et al., 2007; Baroncelli et al., 2010b), enhancing neuronal plasticity in the adult brain. Thus, EE could be particularly suitable for the treatment of pathologies linked to disruption of the GABAergic tone. Given that results obtained in postmortem tissue suggest that people with DS have an impaired balance between excitatory and inhibitory systems (see Rissman and Mobley, 2011 for a recent review), here we choose to investigate the effects elicited by EE on Ts65Dn mice, the DS model in which this alteration has been characterized most extensively. We report that EE reduces GABA release in the hippocampus and the visual cortex of Ts65Dn mice and is highly effective in favoring recovery of spatial learning, hippocampal LTP, and visual acuity.
Materials and Methods
All the procedures employed in this study were approved by the Italian Ministry of Public Health.
Animals
Segmental trisomy 16 (Ts65Dn) mice (Jax West Laboratories, Davis, CA) were obtained by mating female carriers of the 1716 chromosome (B6EiC3H – a/ATs65Dn) with (C57BL/6JEi × C3H/HeJ)F1 (JAX # JR1875) males. Ts65Dn mice were thus maintained on the B6/C3H background. Wild-type (WT) (euploid littermates of Ts65Dn mice) and Ts65Dn mice arriving from Jax Laboratories were housed in plexiglas cages, kept on a 12 h/12 h light-dark cycle and acclimated to these controlled housing conditions for at least one week before inclusion in the study. A total of 39 WT and 44 Ts65Dn mice of both genders were used in this study. In all experiments we included in the analysis both WT and trisomic pups of the same litters.
Genotyping
All WT and Ts65Dn mice were genotyped using real-time quantitative PCR (qPCR) with TaqMan probes (Applied Biosystems) specific for amyloid ß precursor protein gene (App), apolipoprotein B gene (Apob), and myxovirus resistance 1 gene (Mx1). The sequences of primers and probes were as follows:
- ApoB Probe: 5′-CCAATGGTCGGGCACT-3′; ApoB F: 5′CACGTGGGCTCCAGCATT-3′; ApoB R: 5′-TCACCAGTCA TTTCTGCCTTTG-3′;
- App Probe: 5′-CCATCATCGGACTCAT-3′; App F: 5′TGCTGAAGATGTGGGTTCGA-3′; App R: 5′-GACAATCA CGGTTGCTATGACAA-3′;
- Mx1 Probe: 5′-TGGCTTTCCTGGTCGC-3′; Mx1 F: 5′-TCTCCGATTAACCAGGCTAGCTAT-3′; Mx1 R: 5′-GACA TAAGGTTAGCAGCTAAAGGATCA-3′
Genes for App and Mx1 were used as the target (marker) genes, whereas the ApoB gene was used as the internal control. The rationale is that App and Mx1 are located in the chromosome segment triplicated in Ts65Dn, whereas ApoB is mapped on chromosome 12. Thus, Ts65Dn samples have three copies of App and Mx1, whereas euploid samples have only two copies. Both Ts65Dn and euploid animals have two copies of ApoB. The extra copy of App or Mx1 in trisomic samples is detected by qPCR. After amplification, the average change in cycle threshold (ΔΔCT) of the target gene App/Mx1 from that of ApoB in sample animals with respect to controls was calculated (ΔΔCT = (CTApp/Mx1 − CTApoB)trisomic − (CTApp/Mx1 − CTApoB)euploid). The ΔΔCT value for App or Mx1 in trisomic samples is <−0.3.
WT and Ts65Dn mice were also genotyped for the retinal degeneration (rd) allele before being submitted to experimentation; Rd–/– homozygotes were excluded from the study.
Rearing Environments
Adult WT mice of postnatal age > 60 days (P60) were maintained in standard conditions (non-EE) consisting of a standard laboratory cage (26 × 42 × 18 cm) housing a maximum of three females or brother males. Age-matched Ts65Dn mice were reared for six weeks in either EE or in non-EE cages. EE consisted of a large cage (44 × 62 × 28 cm) with a wire mesh lid containing several food hoppers, running wheels, and differently shaped objects (e.g., tunnels, shelters, stairs) that were repositioned twice per week and completely substituted with others once per week. Every EE cage housed at least six females; since it is known that Ts65Dn males have a tendency toward social subordination and that an excess of social stimulation can disturb their behavioral and learning skills (Martínez-Cué et al., 2005), we used a protocol of EE in which only 2–3 Ts65Dn males from the same litter were reared together. Litter and food were the same in both experimental conditions; food and water were available ad libitum.
Morris Water Maze
Mice (n = 9 WT, n = 6 Ts65Dn-non-EE mice and n = 5 Ts65Dn-EE mice) were trained for two trials per day and for a total of 10 days in a circular water tank, made from gray polypropylene (diameter, 100 cm; height, 40 cm), filled to a depth of 25 cm with water (23°C) rendered opaque by the addition of a small amount of atoxic white paint. Four positions around the edge of the tank were arbitrarily designated North (N), South (S), East (E), and West (W), which provided four alternative start positions and also defined the division of the tank into four quadrants: NE, SE, SW, and NW. To avoid possible confounding effects due to reduced visual acuity in Ts65Dn mice, the tank was surrounded by a set of extra-maze cues in the visual discrimination range detectable by all the three groups. A circular clear Perspex escape platform (diameter, 10 cm; height, 2 cm) was submerged 0.5 cm below the water surface and placed at the midpoint of one of the four quadrants. The hidden platform remained in the same quadrant during training, while the start positions (N, S, E, or W) were randomized across trials. Mice were allowed up to 60 s to locate the escape platform, and their escape latency was automatically recorded by the Noldus Ethovision system. On the last trial of the last training day, mice received a single probe trial, during which the escape platform was removed from the tank and the swimming paths were recorded over 60 s while mice searched for the missing platform; the swimming paths were recorded and analyzed with the Noldus Ethovision system.
LTP Recordings
Brains were rapidly removed and immersed in ice-cold cutting solution containing (in mM): 130 NaCl, 3.1 KCl, 1.0 K2HPO4, 4.0 NaHCO3, 5.0 dextrose, 2.0 MgCl2, 1.0 CaCl2, 10 HEPES, 1.0 ascorbic acid, 0.5 myo-Inositol, 2.0 pyruvic acid, and 1.0 kynurenate, pH 7.3. Transverse slices (400 μm thick) from the mid to temporal pole of both hippocampi were obtained using a tissue chopper. Slices (n = 12 slices and eight animals for WT, n = 13 slices and seven animals for Ts65Dn-non-EE mice, and n = 10 slices and five animals for Ts65Dn-EE mice) were allowed to equilibrate at room temperature for at least 1.5 h before being transferred to a recording interface chamber and perfused at a rate of 4 ml/min with 30°C oxygenated recording solution. The recording solution was composed as the cutting solution with the following differences (in mM): 1.0 MgCl2, 2.0 CaCl2, 0.01 glycine, and no kynurenate. Electrical stimulation (100 μsec duration) was delivered with a bipolar concentric stimulating electrode (FHC, St. Bowdoinham, ME) placed in the middle third of the molecular layer ~400 μm from the recording electrode. Dendritic field excitatory post-synaptic potentials (fEPSPs) were recorded by a micropipette (1–3 MΩ) filled with the recording solution and positioned in the middle third of the molecular layer. Baseline responses were obtained every 30 s with a stimulation intensity that yielded a half-maximal response. After achievement of a 15 min stable baseline (field potential amplitude within 15% of change and with no evident increasing or decreasing trends), LTP was induced by a high frequency stimulation (HFS) consisting of four 100 Hz, 0.5 s trains each separated by 30 s. Field recordings were filtered and digitized with an A/D board (National Instruments) driven by a custom acquisition software. LTP graphs were generated by averaging the amplitude of the fEPSPs in 2 min bins, and expressing data as a percentage of the averaged baseline collected before LTP induction. We evaluated field potential potentiation for each group by comparing the last 20 min post-HFS to baseline.
In Vivo Electrophysiology
Mice (n = 13 WT, n = 6 Ts65Dn-non-EE mice and n = 5 Ts65Dn-EE mice) were anesthetized by i.p. injection with Zoletil-100 (40 mg/kg, Virbac), and Xilor (10 mg/kg, Sigma) and placed in a stereotaxic frame. Additional doses of the anesthetic were used to keep the anesthesia level stable throughout the experiment. Body temperature was continuously monitored and maintained at ~37°C by a thermostated electric blanket. A hole was drilled in the skull, corresponding to the binocular portion of the primary visual cortex (V1) (binocular area Oc1B). After exposure of the brain surface, a micropipette filled with NaCl (3 M) was inserted into the cortex 2.8–3.2 mm from λ (intersection between sagittal and lambdoid sutures). Both eyes were fixed and kept open by means of adjustable metal rings surrounding the external portion of the eye bulb. The eyes were frequently inspected and rinsed with physiological solution to prevent the formation of cataracts. Visual acuity through both eyes was measured using visual evoked potentials (VEPs). To record VEPs, the electrode was advanced at a depth of 100 or 400 μm within the cortex. At these depths, VEPs had their maximal amplitude. Signals were band-pass-filtered (0.1–100 Hz), amplified, and fed to a computer for analysis. At least 50 events were averaged in synchrony with the stimulus contrast reversal. Transient VEPs in response to abrupt contrast reversal (1 Hz) were evaluated in the time domain by measuring the peak-to-baseline amplitude and peak latency of the major positive (at 100 μm depth) or negative (at 400 μm depth) component. Visual stimuli were horizontal sinusoidal gratings of different spatial frequencies, generated by a VSG2/2 card running custom software and presented on a monitor (20 × 22 cm; luminance 15 cd/m2) positioned 20 cm from the mouse eyes. Visual acuity was obtained by extrapolation to zero amplitude of the linear regression through the data points in a curve where VEP amplitude is plotted against log spatial frequency. Binocularity was assessed calculating the contralateral to ipsilateral (C/I) VEP ratio at 0.05 cycles per degree (c/deg), i.e., the ratio of VEP amplitudes recorded by stimulating the contralateral and ipsilateral eye with respect to the brain side where recording is performed. For each animal, at least 12 independent C/I VEP ratio values were calculated and averaged together from three well-spaced traces along the medio-lateral and antero-posterior axes of the V1. Care was taken to equally sample VEPs across the two cortical depths so that all layers contributed to the analysis.
Analysis of GABA Release in Hippocampal and Visual Cortex Synaptosomes
Animals (n = 16 WT, n = 10 Ts65Dn-non-EE mice and n = 7 Ts65Dn-EE mice) were sacrificed and the brain regions corresponding to the V1 and to the hippocampus were removed. Synaptosomes were prepared essentially as previously described (Stigliani et al., 2006). The tissue was homogenized at 4°C, utilizing a homogenizer Teflon/glass (clearance 0.25 mm), in 10 volumes of sucrose 0.32 M, buffered with Tris-HCl at pH 7.4. The homogenized tissue was centrifuged (5 min,1000 × g a 4°C) in order to remove all nuclei and cellular fragments. Then, the supernatant was gently stratified on a discontinuous Percoll gradient (2, 6, 10, 20% v/v in tris HCl/sucrose) and again centrifuged (33,500 × g per 5 min a 4°C). After centrifugation, the stratified fraction of synaptosomes, leaning between 10% and 20% Percoll, was collected, washed by centrifugation (20,200 × g per 15 min a 4°C), and then resuspended in a physiologic medium, containing: NaCl 140 mM; KCl 3 mM; MgCl2 1.2 mM; CaCl2 1.2 mM; NaH2PO4 1.2 mM; HEPES 10 mM; glucose 10 mM; pH 7.4.
Synaptosomes were incubated at 37°C for 15 min with the radioactive tracers [3H]D-GABA, at a final concentration of 0.05 μM (plus 50 μM of the GABA transaminase inhibitor amino-oxyacetic acid). Aliquots of the synaptosomal suspensions were layered on microporous filters at the bottom of a set of parallel superfusion chambers (Superfusion System, Ugo Basile, Comerio, Varese, Italy; Raiteri et al., 1984) maintained at 37°C. Superfusion was started at a rate of 0.5 ml/min with standard medium supplemented with 50 μM amino-oxyacetic acid. After 36 min of superfusion, to equilibrate the system, samples were collected according to the following scheme: one sample collected for 3 min (t = 36–39 min; basal outflow); one sample collected for 6 min (t = 39–45 min; stimulus-evoked release); one sample collected for 3 min (t = 45–48 min; basal outflow after stimulus-evoked release). A 90 s period of stimulation was applied at t = 39 min, after the first sample has been collected. Stimulation of synaptosomes was performed with 15 mM KCl, substituting for equimolar concentration of NaCl. Radioactivity was determined in each sample collected and superfused filters by liquid scintillation counting. Tritium released in each sample was calculated as percentage of the total synaptosomal tritium content at the beginning of the respective sample collection (fractional rate × 100). The stimulus-evoked overflow was estimated by subtracting transmitter content of the two 3 min samples (basal outflow) from release evoked in the 6 min sample collected during and after the depolarization pulse (stimulus-evoked release).
Statistics
All statistical analysis was done using SigmaStat Software. Differences between two groups were assessed with a two-tailed t-test. One-Way ANOVA, Two-Way ANOVA, and Two-Way RM ANOVA, followed by Holm–Sidak multiple comparison procedure, were used to compare data belonging to three groups. Level of significance was p < 0.05, unless otherwise specified. No difference emerged between data obtained in enriched male and female Ts65Dn mice for latency in finding the platform on the last day of training in the Morris water maze (MWM) task (t-test, p = 0.185) and in the time exploring the target quadrant during the probe session (t-test, p = 0.278), for hippocampal level of LTP (Two-Way RM ANOVA, p = 0.256), visual acuity (t-test, p = 0.678), ocular dominance (p = 0.526), and GABA release in brain synaptosomes (Two-Way ANOVA, p = 0.215). Since male and female data did not differ, they have been combined together for further analysis.
Results
Spatial Memory
We first assessed spatial memory abilities in the MWM task, a cognitive paradigm in which Ts65Dn mice are known to be severely impaired (Escorihuela et al., 1995; Stasko and Costa, 2004; Fernandez et al., 2007). We found that the while the latency to locate the submerged platform on the ninth day of training was longer in Ts65Dn mice reared in standard environmental conditions (Ts65Dn-non-EE mice, 33.02 ± 8.24 s) compared to both WT (14.52 ± 3.34 s) and Ts65Dn enriched mice (Ts65Dn-EE mice, 15.61 ± 2.53 s), these two latter groups did not differ between each other (Figure 1A). To assess the strength of spatial learning we performed a probe trial in which the hidden platform was removed and the amount of time spent in the former region of platform was measured. The probe test confirmed the spatial memory impairment of Ts65Dn mice: WT mice spent significantly longer time in the quadrant where the platform was located during the previous learning days; in contrast, Ts65Dn-non-EE mice showed no preference for the target quadrant (Figure 1B), indicating that they did not remember the location of the hidden platform. Also in this case, EE was able to completely counteract the cognitive deficit displayed by Ts65Dn mice (Figure 1B).
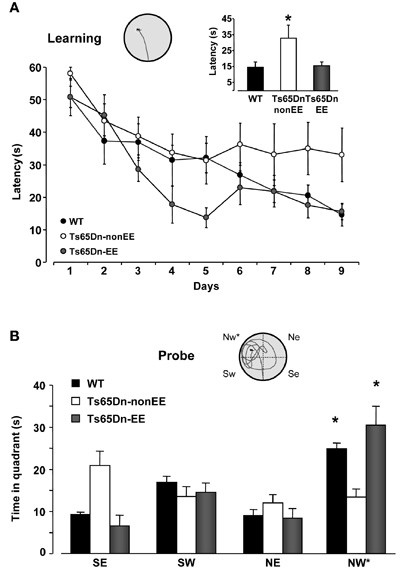
Figure 1. Environmental enrichment promotes spatial learning in Ts65Dn mice. (A) Learning curves for WT (black) and Ts65Dn mice maintained in standard (Ts65Dn-non-EE, white) or enriched (Ts65Dn-EE, gray) environmental conditions. The histogram shows latency to locate the submerged platform on the last day of training for the three groups. One-Way ANOVA followed by a multiple comparison procedure (Holm–Sidak method) showed a statistical difference between WT and Ts65Dn-non-EE mice, but not between WT and Ts65Dn-EE mice (p = 0.885). Inset is an example of swimming path during the last day of training for a Ts65Dn-EE mouse. (B) Probe trial. Two-Way RM ANOVA revealed a statically significant interaction between the genotype group and the pool quadrant (p < 0.001). A Holm–Sidak multiple comparison procedure revealed that while Ts65Dn-non-EE did not show any preference for the target (NW) quadrant, both WT and Ts65Dn-EE mice spent significantly more time in the NW quadrant than in the other quadrants. Moreover, the time spent in the target quadrant was shorter in Ts65Dn-non-EE mice than in the other two groups, which instead did not differ between each other (p = 0.1). Inset shows an example of swimming path during the probe session for a Ts65Dn-EE mouse.
* statistical significance. Error bars, s.e.m.
Synaptic Plasticity in the Hippocampus
Since the spatial memory impairment displayed by Ts65Dn mice has been repeatedly related to synaptic plasticity deficits in the hippocampus (e.g., Siarey et al., 1997, 1999; Kleschevnikov et al., 2004; Fernandez et al., 2007), we studied LTP in the dentate gyrus in response to HFS of the perforant path, the circuitry most severely affected by excessive levels of inhibition in the Ts65Dn mouse brain (Belichenko et al., 2004; Fernandez et al., 2007; Kleschevnikov et al., 2011). While we found a strong potentiation of the response after HFS in the dentate gyrus of WT mice (132.04 ± 7.13%), no potentiation of fEPSPs with respect to baseline was recorded in Ts65Dn-non-EE mice (105.38 ± 5.85%). In contrast, a robust rescue of LTP was evident in Ts65Dn-EE mice (124.5 ± 7.67%) (Figure 2). These results demonstrate for the first time that EE in adult Ts65Dn mice can restore long-term synaptic plasticity in a neural circuit critically involved in spatial learning and memory.
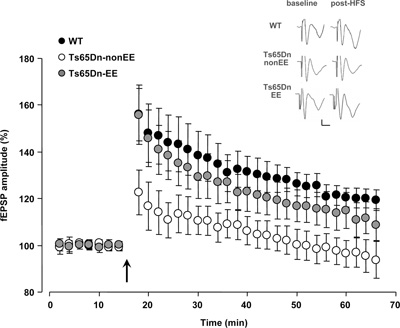
Figure 2. Recovery of long-term potentiation (LTP) at medial perforant path–granule cell synapses in Ts65Dn mice reared in environmental enrichment. Averaged data for LTP induced in WT (black circles), Ts65Dn-non-EE (white circles) and Ts65Dn-EE mice (gray circles). Only slices from WT and Ts65Dn-EE mice showed potentiation of the response after high frequency stimulation (HFS) (Two-Way RM ANOVA, baseline vs. the last 20 min post-HFS, p < 0.001), while slices from Ts65Dn-non-EE did not show potentiation (Two-Way RM ANOVA, baseline vs. the last 20 min post-HFS, p = 1). A multiple comparison procedure (Holm–Sidak method) showed a statistical difference in LTP levels between WT and Ts65Dn-non-EE mice, between Ts65Dn-non-EE mice and Ts65Dn-EE mice and between WT and Ts65Dn-EE mice (p < 0.001). Sample traces (vertical scale bar: 200 μV; horizontal scale bar: 2 ms) from the three experimental groups before (baseline) and after HFS (post-HFS) are also reported. The arrow indicates HFS.
* statistical significance; error bars, s.e.m.
Visual Functions
It has been recently reported that Ts65Dn mice exhibit a number of visual deficits similar to those reported in individuals with DS (Scott-McKean et al., 2010). Therefore, we moved to the visual system with the aim to investigate whether the beneficial effects exerted by EE are specific to the hippocampus or they are also detectable in the sensory cortices. Using electrophysiological recordings of VEPs from the V1, we measured visual acuity of WT, Ts65Dn-non-EE, and Ts65Dn-EE mice and, for the first time in this model, we analyzed the ocular dominance properties of visual cortical neurons calculating the C/I VEP ratio as an index of V1 binocularity (see Sale et al., 2007). Visual acuity of Ts65Dn-non-EE mice was lower (0.45 ± 0.04 c/deg) than that of WT controls (0.57 ± 0.02 c/deg) (Figure 3A). While it is well known that the C/I VEP ratio is in the 2.0–3.0 range in adult normal mice (Porciatti et al., 1999), reflecting the predominance of crossed fibers in retinal projections, we found that the visual cortex of Ts65Dn-non-EE mice was not dominated by the contralateral eye, with a marked reduction in C/I VEP ratio compared to WT animals (Figure 3B; C/I VEP ratio of Ts65Dn-non-EE mice: 1.26 ± 0.18; C/I VEP ratio, WT mice: 2.36 ± 0.16). Moreover, we also report, in agreement with data from patients with DS, that Ts65Dn-non-EE mice did also display a robust increase in VEP latencies compared to WT controls in response to visual gratings of 0.05, 0.1, 0.2, 0.3, and 0.4 spatial frequencies (Figure 3C) (WT 0.05: 125.99 ± 3.28 ms; 0.1: 130.56 ± 5.92 ms; 0.2: 146.24 ± 9.26 ms; 0.3: 144.18 ± 8.85 ms; 0.4: 147.24 ± 8.72 ms. Ts65Dn-non-EE 0.05: 132.79 ± 9.51 ms; 0.1: 145.33 ± 16.38 ms; 0.2: 152.42 ± 8.04 ms; 0.3: 161.03 ± 4.12 ms; 0.4: 168.27 ± 12.89 ms).
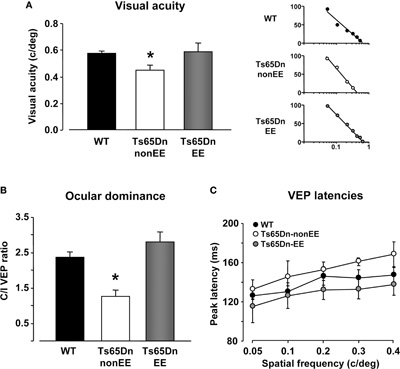
Figure 3. Restoration of visual functions in Ts65Dn mice by environmental enrichment. Visual acuity (A), ocular dominance (B) and peak latency (C) assessed by electrophysiological recordings of visual evoked potentials (VEPs) from the primary visual cortex in WT (black), Ts65Dn-non-EE, (white), and Ts65Dn-EE (gray) mice. One-Way ANOVA showed that a statistical difference in the mean values was present among the three groups for both visual acuity and C/I VEP ratio (p < 0.05 and p < 0.001, respectively); a multiple comparison procedure (Holm–Sidak method) showed a statistical difference between WT and Ts65Dn-non-EE mice and between Ts65Dn-non-EE mice and Ts65Dn-EE mice, but not between WT and Ts65Dn-EE mice (p = 0.82 for visual acuity; p = 0.14 for C/I VEP ratio). VEP latencies of Ts65Dn-EE mice were statistically different from Ts65Dn-non-EE animals, but not from WT mice (Two-Way RM ANOVA). Representative examples of electrophysiological visual acuity assessment of the three experimental groups are also reported on the right of the (a) panel. Visual acuity was obtained by extrapolation to zero amplitude of the linear regression through the data points in a curve where VEP amplitude is plotted against log spatial frequency.
* statistical significance; error bars, s.e.m.
EE completely reversed all visual function deficits displayed by Ts65Dn mice (Figures 3A–C): the visual acuity (0.59 ± 0.06) and the C/I VEP ratio (2.81 ± 0.27) of Ts65Dn-EE mice, indeed, were not statistically different from those of WT mice; in addition, we found that VEP latencies in Ts65Dn-EE mice were significantly shorter than of Ts65Dn-non-EE mice, but did not differ from those recorded in WT controls (Figure 3C) (Ts65Dn-EE 0.05: 115.35 ± 16.50 ms; 0.1: 126.33 ± 12.83 ms; 0.2: 132.25 ± 9.56 ms; 0.3: 132.93 ± 10.04 ms; 0.4: 137.30 ± 10.83 ms).
GABA Release in the Hippocampus and in the Visual Cortex
Because substantial evidence suggests that excessive inhibition is critically involved in the cognitive deficits displayed by Ts65Dn mice (Siarey et al., 1997, 1999; Kleschevnikov et al., 2004; Fernandez et al., 2007), we investigated whether restoration of spatial learning abilities and dentate gyrus long-term plasticity elicited by EE was accompanied by a reduced GABA release in the hippocampus. Specifically, KCl-evoked release of [3H]GABA from synaptosomes in superfusion was measured in the hippocampus of WT, Ts65Dn a-non-EE and Ts65Dn-EE mice (Figure 4A). Moreover, since we previously showed that EE is able to reinstate plasticity in the visual cortex of adult animals through a reduction of GABAergic inhibition (Sale et al., 2007; Baroncelli et al., 2010b), we also performed synaptosome analysis for GABA release in the V1 of the same animals (Figure 4B). We found that the stimulus-evoked release of GABA was markedly increased in both the hippocampus and the visual cortex of Ts65Dn-non-EE mice (hippocampus: 7.30 ± 0.85%; visual cortex: 10.63 ± 0.85%) compared to WT animals (hippocampus: 4.71 ± 0.67%; visual cortex: 7.26 ± 0.90%).
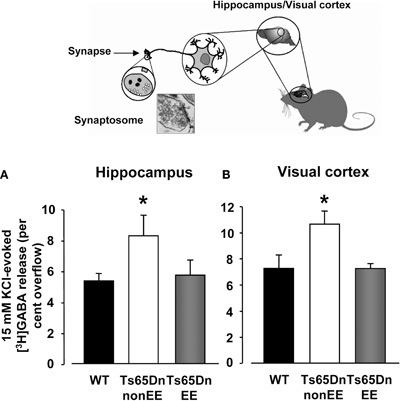
Figure 4. Depolarization-evoked release of GABA from synaptosomes. Insert depicts the synaptosome technique. 15 mM KCl evoked GABA release from hippocampal (A) and visual cortex (B) synaptosomes of wild-type (WT, black), Ts65Dn-non-EE (white) and Ts65Dn-EE (gray) mice. Two-Way ANOVA showed a significant difference among the different levels of group (p = 0.001); a multiple comparison procedure (Holm–Sidak method) showed that levels of GABA were significantly higher, for both the hippocampus and the visual cortex, in Ts65Dn non-enriched mice compared to WT animals, while no statistical difference was present between Ts65Dn enriched animals and WT mice (p = 0.79 for the hippocampus, p = 0.98 for the visual cortex).
* statistical significance; error bars, s.e.m.
In contrast, levels of depolarization-evoked overflow of GABA were markedly decreased in Ts65Dn-EE animals (hippocampus: 5.04 ± 1.02%; visual cortex: 7.22 ± 1.20%) and did not statistically differ from those of WT mice.
Discussion
Our findings show that EE can be successfully employed to favor recovery from cognitive impairment, synaptic plasticity failure, and visual deficits in the most characterized mouse model of DS, the Ts65Dn line. Moreover, we also report that levels of GABA release are markedly increased in the hippocampus and in the visual cortex of Ts65Dn mice compared to WT animals, and that exposure to EE reduces the inhibitory transmission, bringing GABA release in the synaptosomes of trisomic mice back to WT control levels.
These results provide support to the idea that excessive inhibition is a cellular mechanism at the core of the pathogenesis of DS (Fernandez and Garner, 2007). To date, enhanced levels of inhibition in Ts65Dn mice have been mainly documented electrophysiologically. A main finding of the present work is to provide an independent evidence of excessive GABAergic transmission in the hippocampus of Ts65Dn mice using the biochemical model of synaptosome neurotransmitter release analysis. Furthermore, given that increased levels of depolarization-evoked overflow of GABA were also found in the V1, our results suggest that excessive GABAergic transmission might be a more general feature of the Ts65Dn mouse brain, offering an attractive explanation for a number of other functional deficits characterizing DS.
We also showed, in agreement with recent data by Scott-McKean et al., (2010), a robust visual acuity decrease in Ts65Dn mice. Considering that in people with DS the use of corrective lenses does not eliminate major part of their visual deficits (John et al., 2004), it remains unknown whether in addition to anatomical eye abnormalities leading to refractive errors (myopia, for instance, is more common in persons with DS than in the general population; e.g., Shapiro and France, 1985; Liyanage and Barnes, 2008), more subtle functional changes are present at the level of V1 neural connections. In favor of this latter possibility, we report for the first time an alteration in V1 ocular dominance measured in Ts65Dn mice using calculation of C/I VEP ratio. This alteration is reminiscent of the effects elicited by amblyopia (lazy eye) (Holmes and Clarke, 2006), a condition frequently reported in people with DS (Tsiaras et al., 1999) leading to poor visual acuity and successfully revertible in adult rodents by enhancing brain plasticity through exposure to EE conditions (Sale et al., 2007). Therefore, it is possible that the increased environmental stimulation provided by EE might compensate the functional deficits of Ts65Dn mice through a potentiation of neuronal plasticity processes. In line with this interpretation, Ts65Dn mice reared in EE conditions displayed a marked reduction of GABA levels in the visual cortex, a factor crucially involved in the reopening of cortical plasticity in the adult brain (Sale et al., 2007; Maya Vetencourt et al., 2008; Spolidoro et al., 2009; Harauzov et al., 2010).
Our results clearly indicate the possibility to ameliorate, in adulthood, neurological phenotypes associated with early neurodevelopmental disorders, a concept that is attracting a large interest for its potential in clinical application (Ehninger et al., 2008; Silva and Ehninger, 2009; Sale et al., 2010). Reducing inhibition levels with pharmacological treatments has already been shown to promote recovery from cognitive impairment in Ts65Dn mice (Fernandez et al., 2007; Rueda et al., 2008). However, concerns can be raised on the effective clinical utility of GABAA receptor antagonists in persons with DS. As thoroughly discussed by Fernandez et al. (2007), indeed, some drugs (e.g., picrotoxin) are of very limited utility for their pro-convulsive action, a condition particularly dangerous for DS patients, which are more prone to convulsions (see Menendez, 2005), while others, such as bilobalide or pentylenetetrazole, have not been approved by the FDA. EE has the great advantage of reducing inhibition avoiding the negative side effects induced by pharmacological treatments.
Conclusions
The present data indicate how dramatic can be the influence exerted by environmental conditions on brain and behavior. The EE capability to affect GABA release and to promote cognitive and sensory function recovery in a totally non-invasive way makes this paradigm eligible for application in the field of DS therapy. It remains an open issue to what extent is EE in animal models relevant for the human being experience. Although most humans do experience a high degree of environmental complexity and novelty, levels of cognitive, social, and physical stimulation can vary greatly among individuals, particularly in subjects affected by neurological diseases. Our results encourage the development of intervention protocols based on enriched experience aimed at promoting an active lifestyle in DS people.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Research supported by Regione Toscana (Regional Health Research Program 2009) and Fondazione Cassa di Risparmio di Pisa.
References
Baamonde, C., Martínez-Cué, C., Flórez, J., and Dierssen, M. (2011). G-Protein-associated signal transduction processes are restored after postweaning environmental enrichment in Ts65Dn, a Down syndrome mouse model. Dev. Neurosci. 33, 442–450.
Baroncelli, L., Braschi, C., Spolidoro, M., Begenisic, T., Maffei, L., and Sale, A. (2011). Brain plasticity and disease: a matter of inhibition. Neural Plast. 2011, 286073.
Baroncelli, L., Braschi, C., Spolidoro, M., Begenisic, T., Sale, A., and Maffei, L. (2010a). Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ. 17, 1092–1103.
Baroncelli, L., Sale, A., Viegi, A., Maya Vetencourt, J. F., De Pasquale, R., Baldini, S., and Maffei, L. (2010b). Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp. Neurol. 226, 100–109.
Belichenko, P. V., Kleschevnikov, A. M., Masliah, E., Wu, C., Takimoto-Kimura, R., Salehi, A., and Mobley, W. C. (2009). Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down syndrome. J. Comp. Neurol. 512, 453–466.
Belichenko, P. V., Masliah, E., Kleschevnikov, A. M., Villar, A. J., Epstein, C. J., Salehi, A., and Mobley, W. C. (2004). Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J. Comp. Neurol. 480, 281–298.
Blank, M., Fuerst, P. G., Stevens, B., Nouri, N., Kirkby, L., Warrier, D., Barres, B. A., Feller, M. B., Huberman, A. D, Burgess, R. W., and Garner, C. C. (2011). The Down syndrome critical region regulates retinogeniculate refinement. J. Neurosci. 31, 5764–5776.
Brown, J. H., Johnson, M. H., Patterson, S. J., Gilmore, R., Longhi, E., and Karmiloff-Smith, A. (2003). Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia 41, 1037–1046.
Chakrabarti, L., Scafidi, J., Gallo, V., and Haydar, T. F. (2011). Environmental enrichment rescues postnatal neurogenesis defect in the male and female Ts65dn mouse model of Down syndrome. Dev. Neurosci. 33, 428–441.
Clark, D., and Wilson, G. N. (2003). Behavioral assessment of children with Down syndrome using the Reiss psychopathology scale. Am. J. Med. Genet. A. 118, 210–216.
Dierssen, M., Benavides-Piccione, R., Martínez-Cué, C., Estivill, X., Florez, J., Elston, G. N., and DeFelipe, J. (2003). Alterations of neocortical pyramidal cell phenotype in the Ts65Dn mouse model of Down syndrome: effects of environmental enrichment. Cereb. Cortex 13, 758–764.
Ehninger, D., Li, W., Fox, K., Stryker, M. P., and Silva, A. J. (2008). Reversing neurodevelopmental disorders in adults. Neuron 60, 950–960.
Escorihuela, R. M., Fernandez-Teruel, A., Vallina, I. F., Baamonde, C., Lumbreras, M. A., Dierssen, M., Tobena, A., and Florez, J. (1995). A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci. Lett. 199, 143–146.
Fernandez, F., and Garner, C. C. (2007). Over-inhibition: a model for developmental intellectual disability. Trends Neurosci. 30, 497–503.
Fernandez, F., Morishita, W., Zuniga, E., Nguyen, J., Blank, M., Malenka, R. C., and Garner, C. C. (2007). Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat.Neurosci. 10, 411–413.
Gardiner, K., Fortna, A., Bechtel, L., and Davisson, M. T. (2003). Mouse models of Down syndrome: how useful can they be? Comparison of the gene content of human chromosome 21 with orthologous mouse genomic regions. Gene 318, 137–147.
Harauzov, A., Spolidoro, M., DiCristo, G., De Pasquale, R., Cancedda, L., Pizzorusso, T., Viegi, A., Berardi, N., and Maffei, L. (2010). Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J. Neurosci. 30, 361–371.
Holtzman, D. M., Santucci, D., Kilbridge, J., Chua-Couzens, J., Fontana, D. J., Daniels, S. E., Johnson, R. M., Chen, K., Sun, Y., Carlson, E., Alleva, E., Epstein, C. J., and Mobley, W. C. (1996). Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 93, 13333–13338.
Hunter, C. L., Bimonte, H. A., and Granholm, A. C. (2003). Behavioral comparison of four and six month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav. Brain Res. 138, 121–131.
John, F. M., Bromham, N. R., Woodhouse, J. M., and Candy, T. R. (2004). Spatial vision deficits in infants and children with Down syndrome. Invest. Ophthalmol. Vis. Sci. 45, 1566–1572.
Kleschevnikov, A. M., Belichenko, P. V., Gall, J., George, L., Nosheny, R., Maloney, M. T., Salehi, A., and Mobley, W. C. (2011). Increased efficiency of the GABAA and GABAB receptor-mediated neurotransmission in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. [Epub ahead of print].
Kleschevnikov, A. M., Belichenko, P. V., Villar, A. J., Epstein, C. J., Malenka, R. C., and Mobley, W. C. (2004). Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J. Neurosci. 24, 8153–8160.
Lejeune, J., Gautier, M., and Turpin, R. (1959). Etude des chromosomes somatiques de neuf enfants mongoliens. C. R. Hebd. Seances Acad. Sci. 248, 1721–1722.
Liyanage, S., and Barnes, J. (2008). The eye and Down's syndrome. Br. J. Hosp. Med. (Lond.) 69, 632–634.
Martínez-Cué, C., Baamonde, C., Lumbreras, M., Paz, J., Davisson, M. T., Schmidt, C., Dierssen, M., and Florez, J. (2002). Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behav. Brain Res. 134, 185–200.
Martínez-Cué, C., Rueda, N., Garcia, E., Davisson, M. T., Schmidt, C., and Florez, J. (2005). Behavioral, cognitive and biochemical responses to different environmental conditions in male Ts65Dn mice, a model of Down syndrome. Behav. Brain Res. 163, 174–185.
Maya Vetencourt, J. F., Sale, A., Viegi, A., Baroncelli, L., De Pasquale, R., O'Leary, O. F., Castren, E., and Maffei, L. (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388.
Moore, C. S., and Roper, R. J. (2007). The power of comparative and developmental studies for mouse models of Down syndrome. Mamm. Genome 18, 431–443.
Nadel, L. (2003). Down's syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2, 156–166.
Nithianantharajah, J., and Hannan, A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709.
Pennington, B. F., Moon, J., Edgin, J., Stedron, J., and Nadel, L. (2003). The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child. Dev. 74, 75–93.
Perez-Cremades, D., Hernandez, S., Blasco-Ibanez, J. M., Crespo, C., Nacher, J., and Varea, E. (2010). Alteration of inhibitory circuits in the somatosensory cortex of Ts65Dn mice, a model for Down's syndrome. J. Neural Transm. 117, 445–455.
Porciatti, V., Pizzorusso, T., and Maffei, L. (1999). The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res. 39, 3071–3081.
Raiteri, M., Bonanno, G., Marchi, M., and Maura, G. (1984). Is there a functional linkage between neurotransmitter uptake mechanisms and presynaptic receptors? J. Pharmacol. Exp. Ther. 231, 671–677.
Reeves, R. H., Irving, N. G., Moran, T. H., Wohn, A., Kitt, C., Sisodia, S. S., Schmidt, C., Bronson, R. T., and Davisson, M. T. (1995). A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 11, 177–184.
Rissman, R. A., and Mobley, W. C. (2011). Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease. J. Neurochem. 117, 613–622.
Rueda, N., Florez, J., and Martínez-Cué, C. (2008). Chronic pentylenetetrazole but not donepezil treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. Neurosci. Lett. 433, 22–27.
Sale, A., Berardi, N., and Maffei, L. (2009). Enrich the environment to empower the brain. Trends Neurosci. 32, 233–239.
Sale, A., Berardi, N., Spolidoro, M., Baroncelli, L., and Maffei, L. (2010). GABAergic inhibition in visual cortical plasticity. Front. Cell. Neurosci. 4, 10. doi: 10.3389/fncel.2010.00010
Sale, A., Maya Vetencourt, J. F., Medini, P., Cenni, M. C., Baroncelli, L., De Pasquale, R., and Maffei, L. (2007). Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 10, 679–681.
Scott-McKean, J. J., Chang, B., Hurd, R. E., Nusinowitz, S., Schmidt, C., Davisson, M. T., and Costa, A. C. (2010). The mouse model of Down syndrome Ts65Dn presents visual deficits as assessed by pattern visual evoked potentials. Invest. Ophthalmol. Vis. Sci. 51, 3300–3308.
Seregaza, Z., Roubertoux, P. L., Jamon, M., and Soumireu-Mourat, B. (2006). Mouse models of cognitive disorders in trisomy 21: a review. Behav. Genet. 36, 387–404.
Shapiro, M. B., and France, T. D. (1985). The ocular features of Down's syndrome. Am. J. Ophthalmol. 99, 659–663.
Siarey, R. J., Carlson, E. J., Epstein, C. J., Balbo, A., Rapoport, S. I., and Galdzicki, Z. (1999). Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology 38, 1917–1920.
Siarey, R. J., Stoll, J., Rapoport, S. I., and Galdzicki, Z. (1997). Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down syndrome. Neuropharmacology 36, 1549–1554.
Silva, A. J., and Ehninger, D. (2009). Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J. Neurodev. Disord. 1, 150–157.
Spolidoro, M., Sale, A., Berardi, N., and Maffei, L. (2009). Plasticity in the adult brain: lessons from the visual system. Exp. Brain Res. 192, 335–341.
Stasko, M. R., and Costa, A. C. (2004). Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav. Brain Res. 154, 1–17.
Stigliani, S., Zappettini, S., Raiteri, L., Passalacqua, M., Melloni, E., Venturi, C., Tacchetti, C., Diaspro, A., Usai, C., and Bonanno, G. (2006). Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J. Neurochem. 96, 656–668.
Tsiaras, W. G., Pueschel, S., Keller, C., Curran, R., and Giesswein, S. (1999). Amblyopia and visual acuity in children with Down's syndrome. Br. J. Ophthalmol. 83, 1112–1114.
Keywords: Down syndrome, Ts65Dn mice, environmental enrichment, GABAergic inhibition, cerebral plasticity
Citation: Begenisic T, Spolidoro M, Braschi C, Baroncelli L, Milanese M, Pietra G, Fabbri ME, Bonanno G, Cioni G, Maffei L and Sale A (2011) Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome. Front. Cell. Neurosci. 5:29. doi: 10.3389/fncel.2011.00029
Received: 10 November 2011; Accepted: 12 December 2011;
Published online: 23 December 2011.
Edited by:
Arianna Maffei, SUNY Stony Brook, USAReviewed by:
Marcos G. Frank, University of Pennsylvania, USAMelanie A. Woodin, University of Toronto, Canada
Anthony Hannan, University of Melbourne, Australia
Copyright: © 2011 Begenisic, Spolidoro, Braschi, Baroncelli, Milanese, Pietra, Fabbri, Bonanno, Cioni, Maffei and Sale. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Alessandro Sale, Department of Medicine, Institute of Neuroscience National Research Council (CNR), via Moruzzi 1, Pisa I-56124, Italy. e-mail: sale@in.cnr.it
† These authors equally contributed to this work.