-
PDF
- Split View
-
Views
-
Cite
Cite
Kristen R. Senechal, Christina Thaller, Jeffrey L. Noebels, ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy, Human Molecular Genetics, Volume 14, Issue 12, 15 June 2005, Pages 1613–1620, https://doi.org/10.1093/hmg/ddi169
Close - Share Icon Share
Abstract
Mutations in LGI1 have been linked to autosomal dominant partial epilepsy with auditory features (ADPEAF), an unusual inherited human partial epilepsy phenotype. In addition, decreases in LGI1 expression are observed in glioblastoma patient samples and glioblastoma cell lines. LGI1, one member of the LGI gene family, encodes a ∼63 kDa protein, with strong regional expression in neurons within the temporal lobe. Although the function of LGI proteins remains unknown, structural analyses suggest that LGI1 could be either localized to the membrane or secreted. Here, we show that LGI1–4 exhibit overlapping patterns of diffuse mRNA expression in the adult mouse brain, with some areas of specific localization characteristic of each family member. We find robust secretion of mouse LGI1 protein following transfection into 293T cells. LGI family members, LGI3, LGI4 and a newly identified splice form of LGI2, LGI2B, are also secreted in culture, indicating that secretion is a conserved feature of this protein family. Introduction of mutations in LGI1, including those identified in ADPEAF pedigrees, reveals that the mutant proteins either are not secreted or are unstable. These results demonstrate loss-of-function as a pathogenic basis for LGI1-mediated ADPEAF.
DDBJ/EMBL/GenBank accession no. AY841361
INTRODUCTION
The distinctive phenotype of auditory hallucinations and focal seizure localization in the lateral temporal lobe epilepsy syndrome, autosomal dominant partial epilepsy with auditory features (ADPEAF), enabled linkage mapping of a gene locus on chromosome 10q24s, where the leucine-rich glioma inactivated gene-1 (LGI1), encoding a protein of unknown function, was identified as the target for mutation in these families (1–3). LGI1 was previously suggested to function as a tumor suppressor in the brain on the basis of its decreased expression in human glial tumor samples and glioblastoma cell lines when compared with whole brain tissue (4). In addition, LGI1 has been shown to decrease proliferation and in vitro invasion of glioblastoma cell lines (5,6). Such comparisons, while intriguing, are difficult to interpret given the heterogeneity of cell types and differentiation states in whole brain and tumor samples, as well as the transformed nature of tumor-derived cell lines. In fact, subsequent expression studies revealed that the gene is strongly transcribed in neuronal cells but not in healthy glial cells (2).
A second insight into the potential function was suggested by the homology between LGI1 and Slit proteins, which are involved in axonal path finding (4,7–10). However, the percent homology between mammalian Slit and LGI proteins (29–32% identity) lies only within the N-terminal half of LGI1 protein and is restricted to the regions of the leucine-rich repeats (NCBI BLAST). It is unclear whether these structural similarities extend to function and how LGI1 may play a role in tumor biology or CNS development.
There is considerable uncertainty surrounding the subcellular localization of LGI1. Initially, a potential transmembrane region within the protein was identified (4), however, the SMART protein motif prediction program does not identify a transmembrane sequence (smart.embl-heidelberg.de/). Other recent analyses suggest that there is no functional transmembrane domain and that the protein is composed of leucine-rich repeats, surrounded by cysteine-rich regions, all upstream of several epilepsy-associated repeat/Epitempin repeat (EAR/EPTP) domains. EAR/EPTP domains have been postulated to create a beta-propeller structure proposed to function as a protein–protein interaction domain (11,12). The only consensus structural feature related to potential localization is a signal peptide at the N-terminus. At an ultrastructural level, there is currently no direct evidence for or against the membrane localization of LGI1. One report has indicated that LGI1 is present in vesicle-like organelles, but again, direct evidence for membrane localization is lacking (3).
In the current study, we extend the examination of the mRNA expression pattern to LGI1–4 in the adult mouse brain and show that each family member has both overlapping and distinct areas of expression. In vitro analysis of LGI1 by transient transfection analysis in a heterologous system indicates that the majority of LGI1 protein is constitutively secreted and not confined to either intracellular or transmembrane compartments. We further identify a previously unrecognized splice form of LGI2, LGI2B, and show that LGI2B, 3 and 4 all exhibit secretion profiles that are similar to LGI1, suggesting that secretion is intrinsically important to the function of the LGI family. Introduction of mutations into the LGI1 coding sequence, including several mutations identified in inherited human ADPEAF cases, resulted in decreased levels of secreted LGI1 protein, indicating a loss-of-function mechanism for the pathogenesis of LGI1-mediated ADPEAF.
RESULTS
LGI family member mRNAs are expressed in overlapping and distinct areas of the brain
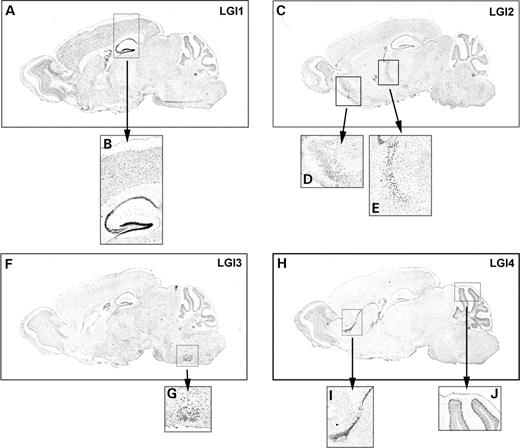
We used in situ hybridization to compare the mRNA expression pattern of each member of the LGI family. Two probes were designed for each mRNA, and in all cases, both probes showed similar patterns of staining using sense probes as controls. The results of the N-terminal probes are shown (Fig. 1). The in situ hybridization data for LGI1 are similar to the pattern previously reported (2), with diffuse staining in the neocortex and other brain regions, along with intense localization in the granule cells of the dentate gyrus and the CA3–CA1 region of the hippocampal pyramidal cell layer (Fig. 1A and B). Hybridization for LGI2 shows low levels of diffuse staining throughout the brain and high levels in the pyriform cortex and the thalamic reticular nucleus (Fig. 1C–E). LGI3, in addition to the low levels of diffuse staining in total brain, is expressed most strongly in the facial nerve nucleus (Fig. 1F and G). Finally, expression of LGI4 mRNA is specifically increased in the purkinje cell layer of the cerebellar cortex and in a pattern corresponding to the rostral migratory stream leading to the olfactory cortex (Fig. 1H–J). The pattern of LGI4 expression in the purkinje cell layer is similar to previous reports, however, it should be noted that the 3′-untranslated region (3′-UTR) of the LGI4 gene locus overlaps with the 3′-UTR of another gene, Fxyd3, on the reverse strand, and as a consequence, the level of LGI4 protein expression may be influenced by the formation of double-stranded RNA molecules in vivo (13). Our LGI4 probe is homologous to the 5′ end of the mRNA and does not hybridize with the Fxyd3 gene, adding further precision to this pattern of localization.
LGI1 is constitutively secreted by 293T cells
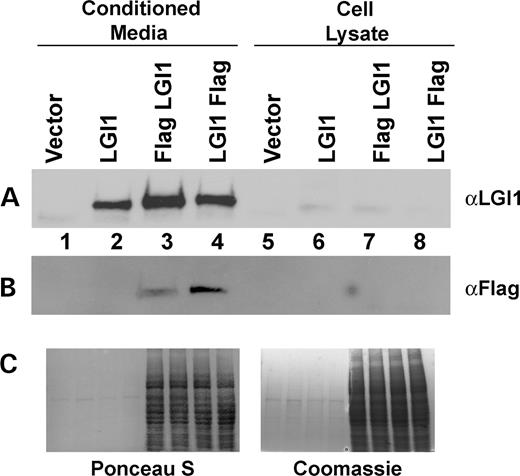
LGI1 cDNA was isolated from adult C57BL/6 mouse brain with primers based on accession no. NM_020278. The Flag epitopes were added either directly to the C-terminus or immediately after the signal peptide cleavage site (KK, amino acids 35 and 36) to tag the N-terminus. The LGI1 cDNAs were transfected into 293T cells and the cultures analyzed 36–48 h after transfection, following 12–16 h of serum-free media incubation. The serum-free incubation was necessary due to the abundance of albumin in the conditioned media that inhibited visualization of the LGI1 protein by western blot, because both proteins are roughly 60–63 kDa. Conditioned media samples were equalized for total protein concentration, as were cell lysate samples and equal proportions of the samples were analyzed, i.e. one-tenth volume of the conditioned media was compared to one-tenth volume of the cell lysate. Ponceau S staining of the filter prior to immunoblotting and Coomassie staining of the samples run in duplicate are shown to demonstrate equivalent protein loading among the conditioned media samples and among the cell lysate samples (Fig. 2C).
High LGI1 protein levels were seen in the conditioned media, visualized with a commercially available LGI1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and LGI1 was not detected at significant levels in the lysate under these exposure conditions (Fig. 2A, lanes 2 and 6). Marginal LGI1 levels could be observed in the lysates, if the lysates were concentrated and the immunoblot exposures were significantly longer, however, the amounts in the lysate were always less than those in the conditioned media (data not shown).
We further determined that the modification of either the N- or the C-terminus by addition of epitope tags did not alter LGI1 secretion. Flag LGI1 and LGI1 Flag proteins were both found in the media, as seen in the LGI1 and Flag M2 immunoblots, but were not significantly detected in the lysates (Fig. 2A and B, lanes 2–4 and 6–8). These results indicate that LGI1 is a secreted protein in 293T cells.
LGI2, 3 and 4 are secreted
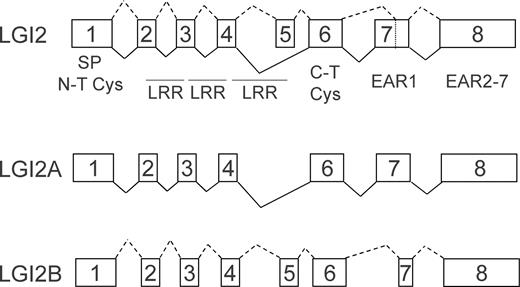
To extend the analysis of LGI protein secretion, LGI2, 3 and 4 cDNAs were generated from adult mouse brain based on sequences for these genes contained in GenBank. Repeated attempts to isolate the murine LGI2 cDNA as described in NCBI (NM_144945) from mouse brain were unsuccessful. The RT–PCR product of LGI2 that was isolated appears to be an alternative splice product of the original LGI2 sequence (Fig. 3). We refer to the original sequence as LGI2A and our clone as LGI2B. LGI2A does not include exon 5 (unlike the structure of family members 1, 3 and 4), whereas LGI2B does include this exon which encodes the C-terminal segment of the last leucine-rich repeat. The remaining LGI2 exons that encode for the regions downstream of the leucine repeats have been renumbered on the basis of the inclusion of exon 5. An additional difference between LGI2A and 2B is alternative splicing within exon 7 (described as Exon 6 in the original 2A sequence). LGI2A utilizes an exon 7 of 261 nt, whereas LGI2B uses an alternative splice site at position 97 of exon 7, resulting in a 165 nt exon which retains the original translational frame.
Mouse LGI3 was also isolated by RT–PCR and was similar to the NCBI sequence (NM_145219). Isolation of full-length LGI4 cDNA was problematic because our full-length PCR cloned products proved to be unstable in several Escherichia coli competent cell strains. Ultimately, Stbl2 cells (Invitrogen, Carlsbad, CA, USA) were successfully utilized to obtain a full-length cDNA clone matching the NCBI sequence (NM_144556).
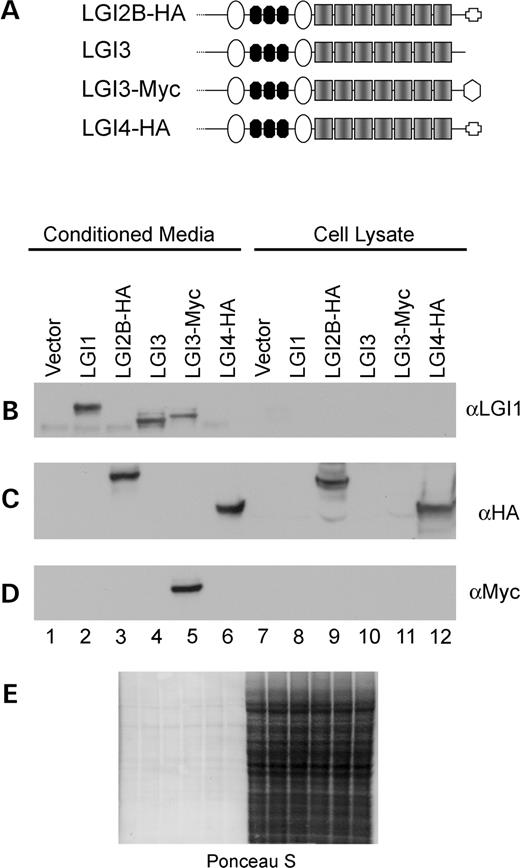
On the basis of sequence similarities between LGI1 and the other family members, we predicted that LGI2, 3 and 4 would also be secreted proteins. To test this, cDNAs for the various constructs, schematically depicted in Figure 4A, were epitope tagged, cloned into a mammalian expression vector and transfected into 293T cells. Conditioned media from each transfection was collected and analyzed in parallel with cell lysates as performed with LGI1.
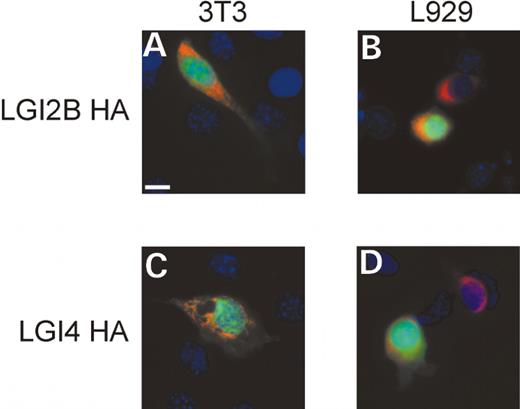
LGI2B was analyzed by immunoblot based on the hemagglutinin tag (HA) that was added to the C-terminus. We found that LGI2B-HA is secreted and is also present to some extent in the cell lysate (Fig. 4C, lanes 3 and 9). The proportion of LGI2B-HA retained in the cell lysate exceeded that observed for LGI1, which may reflect either an intrinsic property or an enhanced stability of the LGI2B protein sequence or interference of efficient secretion of the protein by the epitope tag. The latter is less likely because the epitope tags had no measurable effect on LGI1 protein secretion. To determine the localization of cellular LGI2B-HA, we performed immunofluorescence staining. Two fibroblast cell lines, 3T3 and L929, were cotransfected with expression constructs for LGI2B-HA and green fluorescent protein (GFP). The localization of GFP (green staining) is cytoplasmic, allowing the visualization of transfected cells. LGI2B-HA (red staining) was neither bound to the plasma membrane nor diffusely cytoplasmic but could be visualized in discrete intracellular compartments, consistent with presence within organelles (endoplasmic reticulum, golgi bodies and vesicles) involved in the secretory pathway (Fig. 5A and B).
During the analysis of LGI3 secretion, we observed significant cross reactivity between the LGI1 antibody and the LGI3 antibody (Fig. 4B, lanes 2, 4 and 5). This permitted direct comparison of any effect that the Myc epitope tag might have on secretion. Both LGI3 and LGI3-Myc were efficiently secreted into the media of 293T cells after transfection, to a similar extent as LGI1 (Fig. 4B, lanes 2, 4 and 5 and Fig. 4D, lane 5).
To complete the characterization of secretion within the LGI protein family, LGI4 was epitope tagged with HA epitope and tested. LGI4-HA was observed in both conditioned media and the cell lysate, as shown by immunoblot for the epitope tag (Fig. 4C, lanes 6 and 12). As with LGI2B-HA, the intracellular pool of LGI4A-HA was confined to specific intracellular compartments, consistent with presence within organelles of the secretory pathway (Fig. 5C and D) in transfected 3T3 and L929 fibroblast cells. This result resembles that of LGI2B-HA, suggesting that their secretion mechanisms and kinetics may be similar.
Mutations in LGI1 result in protein instability and defects in secretion
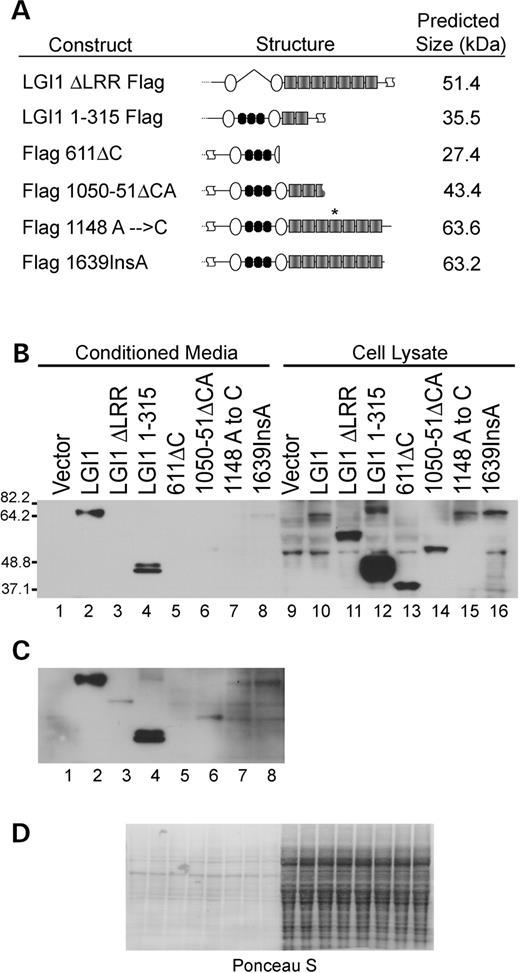
Since the original identification of LGI1 as the pathogenic target in inherited ADPEAF, many additional mutations have been discovered (2,3,14–21). However, the functional consequences of these mutations have not been determined. We introduced several ADPEAF mutations and two others predicted to alter specific structural motifs of the protein into the mouse LGI1 cDNA clone (Fig. 6A) (2). Several of the mutations result in truncations, deleting the LGI1 antibody epitope, therefore, all proteins were Flag epitope tagged. The structural mutations include an in-frame deletion of the leucine-rich repeats, LGI1ΔLRR Flag and LGI1 1–315 Flag, a truncation that removes the C-terminus of the protein including EAR repeats 3 through 7. The ADPEAF mutations that were engineered into the mouse LGI1 clone are 611ΔC (ADPEAF 835delC), 1050–1051ΔCA (ADPEAF 1050–1051delCA), 1148 A→C (ADPEAF 1372 A→C) and 1639InsA (ADPEAF 1639InsA). Each of the ADPEAF-mutant mouse cDNAs was epitope tagged at the N-terminus after the signal peptide cleavage site but before the first cysteine-rich repeat.
293T cells were transfected with expression constructs for each mutation, using a GFP expression plasmid as a control for transfection efficiency. Conditioned media were collected for each sample and cells were lysed as described for secretion analysis of LGI1. In all cases, mutations in the LGI1 sequence decreased the proportion of LGI1 protein present in the conditioned media. The LGI1ΔLRR Flag protein was retained almost exclusively in the cell lysate, unlike Flag LGI1 wild-type (Fig. 6B, lanes 2, 3, 10 and 11). With a longer exposure, LGI1ΔLRR Flag can be observed in the conditioned media but to a lesser degree than in Flag LGI1 wild-type (Fig. 6C, lanes 2 and 3). Deletion of EAR 3–7 in LGI1 1–315 Flag resulted in a majority of the protein remaining intracellular with a small proportion secreted (Fig. 6B, lanes 4 and 12). In addition, this protein appears to be more stable and accumulates to higher intracellular levels than in Flag LGI1. This indicates that the leucine-rich repeats and EARs 3–7 are structurally required for normal levels of secretion in 293T cells. The first ADPEAF mutation analyzed was the Δ611 single base pair deletion. This mutation is predicted to produce a 27.4 kDa protein, deleting the C-terminal end of the C-terminal cysteine-rich repeat and all seven EARs. Flag Δ611 appears larger than its predicted size, which could be due to modifications such as glycosylation. Expression of Flag Δ611 is restricted to the cell lysate and accumulates intracellularly to a greater extent than that of Flag LGI1 (Fig. 6B, lanes 5, 10 and 13). The next mutation, Flag 1050–51ΔCA, was expected to produce a protein with a predicted mass of ∼43 kDa. The higher mass observed for this mutant may also be due to glycosylation or other modifications. Flag LGI1 1050–51ΔCA is retained in the cell lysate (Fig. 6B, lanes 6 and 14) and can only be observed in the conditioned media with prolonged exposures (Fig. 6C, lane 6). This mutant also accumulates within the cell at greater levels than those in Flag LGI1 (Fig. 6B, lanes 10 and 14). The third ADPEAF mutation, Flag 1148 A→C, is postulated to generate a protein similar in size to wild-type LGI1. Flag 1148 A→C is barely detectable in the conditioned media but present in the cell lysate at levels similar to Flag LGI1 wild-type, (Fig. 6B and C, lanes 2, 7, 10 and 15). The last ADPEAF mutation tested, Flag 1639InsA, is also predicted to produce a protein similar in size to LGI1. This mutated protein is expressed in the cell lysate to the same degree as Flag LGI1 wild-type but can be seen in the conditioned media with longer exposure times (Fig. 6B and C, lanes 2, 8, 10 and 16).
DISCUSSION
Mutations in LGI1 underlie a recently isolated inherited developmental brain disorder featuring seizures preceded by a distinctive auditory aura, suggesting a functional disturbance within brain regions mediating higher order processing or aural sensory information. The regional brain localization of LGI1 gene expression within hippocampal and cortical neurons is highly consistent with the partial clinical epilepsy phenotype emanating from this dysfunction of the temporal lobe and surrounding auditory cortex. We now report that LGI1 is a secreted protein and that disease mutations block protein secretion and stability, demonstrating that the clinical epilepsy phenotype likely arises from loss of normal protein function within these developing brain regions.
Using mRNA in situ hybridization, we observed that LGI family members are expressed diffusely throughout the brain with significant areas of overlap, however, each family member exhibited distinct patterns of peak expression in specific regions. Our result showing the localization of LGI1 confirms previously published data in which LGI1 mRNA is intensely expressed in granule cells of the dentate gyrus and the CA3–CA1 pyramidal cell layer of the hippocampus and present in the overlying neocortex, consistent with the clinical temporal lobe epilepsy syndrome associated with mutations in LGI1 (2). Although mutations in LGI2–4 have not yet been observed, their localization to the other specific areas described suggest that functional disturbances in each of these paralogs could yield distinctly different neurological phenotypes.
The protein expression and secretion experiments analyzing LGI1–4 were performed in 293T cells, a non-neuronal cell type, however, the secretion capabilities of this system have made it useful in previous analyses of secretion, clustering and functional localization of neuronal proteins (22–24). On the basis of our findings in 293T cells, LGI1–4 paralogs are all secreted with steady-state intracellular/extracellular protein ratios that vary among the family members. It is of interest that LGI2B and LGI4 are observed in the cell lysate to a greater extent than LGI1 and LGI3, although phylogenetically, LGI2 and 3 are more closely related than LGI2 and 4 are to one another (ClustalW analysis with mouse protein sequences). Using immunofluorescence microscopy, we observed a compartmentalized staining pattern for LGI2B and LGI4, which is consistent with endoplasmic reticulum, golgi bodies and vesicles, suggesting that these family members are retained in intracellular compartments to a greater extent than LGI1 and LGI3. This could be due to various properties including slower kinetics of post-translational modification or a differentially regulated secretion mechanism. Morante-Redolat et al. found that their LGI1 antibody displayed punctate staining in the cell body and neurites and costained with a vesicular protein, Rab3. A vesicular mechanism of transport is consistent with our secretion observations. However, it is unclear whether the antibody utilized by Morante-Redolat et al. is specific for LGI1 or whether there is cross reactivity to other LGI family members, as we observed with the commercially available antibody. Further clarification will require the generation of family member-specific antibodies.
The constitutive secretion of LGI1 raises interesting questions regarding the specific cell types responsible for mutant LGI1 phenotypes. Strong LGI1 mRNA expression is observed by in situ hybridization analysis in the dentate gyrus and pyramidal cell regions of the hippocampus as well as the neocortex (2). However, LGI1 protein expression, as observed by immunohistochemistry, shows a more ubiquitous pattern throughout adult human brain, with much of the staining concentrated in neurons and very little in glial cells (3). Along with evidence for secretion, this apparent mismatch indicates that LGI1 protein may function more globally in the brain than the mRNA expression pattern suggests. However, cross reactivity of antibodies among various family members (as noted in the present study) is likely to complicate immunohistochemical distribution patterns and, therefore, may not be a reliable indicator of specific LGI protein localization or targeting. A search for the receptor and pathways mediating LGI1 activity will be required to define which cells in the brain respond to LGI1 protein and to determine whether the same subpopulations that secrete LGI1 also respond to it.
The data showing that LGI1 is secreted points to an extrinsic mechanism of action. Previous studies utilizing LGI1-transfected glioblastoma cell lines show an inhibitory effect on cell proliferation and in vitro invasion (5,6). On the basis of the present results, we postulate that the transfected cells in those studies secrete LGI1 and that secreted LGI1 may be acting in an autocrine pathway on the cells engineered to express it. The development of resistance to LGI1 signaling in cerebral neoplasms, either by loss-of-function of LGI1 or a potential receptor, would be consistent with the proposed growth suppressor role for this molecule in the normal brain. At this time, the putative LGI1 receptor is unknown, and we cannot directly address the prevalence of the expression of such a protein in any cell line or tissue sample.
In the context of ADPEAF, many families have been identified in which LGI1 is mutated, and a majority of these mutations results in alterations of conserved amino acid residues and truncations. We hypothesized that these mutations would affect the localization of LGI1, specifically, the ability of LGI1 to be secreted and would subsequently result in a loss-of-function disease mechanism. The observations that multiple mutations, including some identified in affected ADPEAF families, alter LGI1 protein secretion and stability are consistent with a loss-of-function model as a mechanism for disease pathogenesis. Although our results do not delineate between defects in the secretion of LGI1 and stability of the mutant proteins once secreted into the extracellular space, a decrease in either property leads to reduced extracellular LGI1 protein.
On the basis of the reported sequence similarity between the LGI proteins and the Slit protein family, an attractive model is that loss of LGI1 function results in altered neuronal populations and/or circuitry during development. Mutations in LGI1 exhibit an autosomal dominant pattern of inheritance and affected family members carry one wild-type allele. This indicates that gene dosage of LGI1 is critical and that reducing normal levels of expression by half can result in an epilepsy phenotype. Together, these observations suggest that LGI1 and family members might function as developmental morphogens or migration cues and may exert their control through the implementation of gradients of the secreted protein in the developing CNS. There has been no speculation of the role of LGI1 in the adult nervous system. Experiments focused on the regulation of neuronal targeting and guidance by LGI1 as well as on the regulation of LGI1 expression in the developing and adult brain will be required to address these issues.
MATERIALS AND METHODS
In situ hybridization and imaging
Primers for RT–PCR and subsequent riboprobe generation were designed by adding T7 and SP6 promoter sequences onto each primer (bold type) to generate riboprobes specific for each family member (lowercase). LGI1-forward: 5′-GCGATTTAGGTGACACTATAGaaagcagcagaaggatgggaaatg, reverse: 5′-GCGTAATACGACTCACTATAGGGagctgcagcgatggtgtgaataag; LGI2-forward: 5′-GCGATTTAGGTGACACTATAGcatcatctgcgtgggctcctctt, reverse: 5′-GCGTAATACGACTCACTATAGggccatttggctttgcagtcacattc; LGI3- forward: 5′-GCGATTTAGGTGACACTATAGccttctcccatctgccgttgttgc, reverse: 5′-GCGTAATACGACTCACTATAGgggcgttgccccggaggtctaagt and LGI4-forward: 5′-gcgatttaggtgacactataggaggggcgggcatcttgttgttc, reverse: 5′-gcgtaatacgactcactatagggggttgttggccaggcttaggtg. RT–PCR was performed on adult mouse brain RNA and PCR products were cloned into the pCRII-TOPO vector (Invitrogen). Digoxigenin-labeled probes were generated with the DIG RNA Labeling Kit (SP6/T7) as described in the manufacturer's instructions (Roche, Indianapolis, IN, USA), purified using Micro Bio-Spin Columns, P-30 Tris RNase-free (BioRad, Hercules, CA, USA) and tested for labeling efficiency using the DIG Nucleic Acid Detection Kit (Roche). In situ analyses and imaging were performed as described (25). In brief, in situ hybridization was performed on 25 µm fresh frozen mouse brain sections. Prehybridization, hybridization, post-hybridization and color reactions were carried out on a Tecan Genesis robotic platform using the GenePaint system (www.genepaint.org).
cDNAs and plasmid constructs
All LGI cDNAs were isolated from C57/Bl6 adult mouse brain. LGI1 was isolated by RT–PCR on the basis of accession no. NM_020278 and cloned into the pcDNA 3.1 (+) vector (Invitrogen). In addition, LGI1 was tagged with the Flag epitope (based on Sigma M2 epitope) by PCR mutagenesis. In one construct the epitope was introduced at the C-terminus of the protein and in a second, the epitope was inserted after the signal peptide between amino acids 36 and 37, but before the first cysteine-rich region. LGI2, 3 and 4 were isolated using primers designed from archived database sequences for these genes (LGI2, NM_144945; LGI3, NM_145219; LGI4, NM_144556). The nucleotide sequence for mouse LGI2B has been deposited in the GenBank database under GenBank accession no. AY841361. The Myc (9E10) epitope was added to the C-terminus of LGI3 via PCR as was the HA tag for LGI2B and LGI4. All cDNAs were sequenced and cloned into the pcDNA 3.1 (+) vector. LGI4 constructs were maintained in the Stbl2 bacterial cell line (Invitrogen) because of the instability of the inserts.
Transfections, media collection, cell lines
293T cells were grown in Dulbecco's modification of Eagle's medium (DMEM) with 10% calf serum (CS) on 6 cm plates and were transfected using FuGene6 (Roche). At 12–16 h before collection and lysis, media were removed and cells were washed twice to remove serum and albumin that reduced visualization of LGI1 by western blot due to size similarity. Cells were then kept in 2 ml serum-free media for 12–16 h. Media were collected and centrifuged to pellet cell debris, and the supernatant/conditioned media were concentrated to 100 µl using Amicon Ultra-4 concentrators (5000 MWCO) and subsequently supplemented with 100 µl 2× lysis buffer [300 mM NaCl, 40 mM Tris, pH 7.5, 20% glycerol, 2% NP-40 and protease inhibitor cocktail (Roche)]. Cells were lysed in 200 µl lysis buffer (150 mM NaCl, 20 mM Tris, pH 7.5, 10% glycerol, 1% NP-40 and protease inhibitor cocktail). Conditioned media samples were equalized for total protein, as were cell lysate samples (Biorad DC Protein Assay). NIH 3T3 and L929 cells were obtained from American Type Culture Collection (ATCC, Manassus, VA, USA). NIH 3T3 cells were grown in DMEM with 10% CS and L929 cells were grown in DMEM with 10% fetal calf serum (FCS), and both lines were incubated in 5% CO2.
Immunoblot, immunofluorescence analysis
Equivalent proportions of conditioned media and lysates were separated on tris–glycine gels (Biorad and Gradipore, New York, NY, USA). Proteins were transferred to nitrocellulose and stained with Ponceau S to show that all conditioned media samples were loaded equal to each other as were the cell lysate samples. In cases where Ponceau S did not adequately stain the low levels of total protein in the conditioned media, samples were separated on a duplicate gel and stained with Coomassie to show equal protein concentration between samples. Ponceau S stained blots were destained and probed using 3% BSA for blocking and incubation with antibodies for the Flag epitope (M2, Sigma-Aldrich, St. Louis, MO, USA), LGI1 (C-19, Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) and HA (Y-11, Santa Cruz). c-Myc (clone 9E10, Roche) immunoblots were blocked and incubated with 5% non-fat dry milk. Immunoblots were developed with ECL and ECL Plus (Amersham Biosciences, Piscataway, NJ, USA). For immunofluorescence experiments, 3T3 and L929 fibroblasts were transfected using FuGene6 (Roche). The pcDNA 3.1 (+) expression vector was used to express the LGI proteins and GFP was expressed from pGFP-C1 (BD Biosciences Clontech, Palo Alto, CA, USA). Transfected cells were fixed 36 h post-transfection with 4% paraformaldehyde and permeabilized with 0.5% Triton ×100 in phosphate-buffered saline. Goat serum was used as a blocking reagent during incubations with the HA antibody (Y-11, Santa Cruz) and secondary antibody (Texas Red conjugated, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Slides were mounted with Vectashield with DAPI (Vector Laboratories, Inc., Burlingame, CA, USA). Cells were visualized and photographed with an Olympus model IX71 inverted microscope (Olympus America, Melville, NY, USA) with epifluorescence. A Hammamatsu Orca CCD camera (Hamamatsu Photonic Systems, Bridgewater, NJ, USA) was used to capture images that were then processed with PCI software (Complix Inc., Cranberry Township, PA, USA) and Adobe Photoshop software (Adobe Systems, Mountain View, CA, USA).
ACKNOWLEDGEMENTS
The authors would like to thank Wayne Ernst and Caleb Davis for technical assistance and advice and Dr Daniel L. Burgess for critical review of the manuscript. This work was supported by NRSA NS045376-03 (KRS), NIH T32-NS43124 (K.R.S.) NINDS NS29709 (JLN), the Blue Bird Circle Foundation, German Ministry of Research 01KW9965 (CT) and the European Union QLRT-1999-00793 (CT). The nucleotide sequence for mouse LGI2B has been deposited in the GenBank database as GenBank accession no. AY84136.
Conflict of Interest statement. None declared.
Figure 1. LGI mRNAs are expressed in overlapping and distinct patterns. Adult mouse brain (P55–60) parasagittal sections were hybridized with digoxigenin-labeled riboprobes for the indicated mRNAs. For each mRNA, two antisense probes were generated and showed the same staining patterns. The N-terminal antisense probes with regions of interest are shown: (A) LGI1, brain; (B) LGI1, cortex and hippocampus; (C) LGI2, brain; (D) LGI2, pyriform cortex; (E) LGI2, thalamic reticular nucleus; (F) LGI3, brain; (G) LGI3, facial nerve nucleus; (H) LGI4, brain; (I) LGI4, rostral migratory stream and (J) LGI4, purkinje cell layer of the cerebellum.
Figure 2. LGI proteins are secreted from 293T cells. Conditioned media were collected and the cells were lysed. Conditioned media samples were equalized to each other on the basis of total protein concentration, as were cell lysate samples, and then equivalent volume proportions of conditioned media and cell lysate were loaded. LGI1 secretion is shown by (A) LGI1 immunoblot and (B) Flag M2 immunoblot. (C) Ponceau S and Coomassie staining are shown to demonstrate equal protein concentration among conditioned media samples and among cell lysate samples.
Figure 3. Digrammatic representation of LGI2A and LGI2B splicing. Solid black lines indicate the LGI2A exon structure and dashed lines indicate the LGI2B exon structure.
Figure 4. LGI family members are secreted from 293T cells. (A) Diagrammatic representation of LGI family member structure and position of epitope tags are denoted by the geometric shapes at the C-termini. In all immunoblotting experiments, conditioned media samples were equalized to each other on the basis of total protein concentration, as were cell lysate samples, and then equivalent volume proportions of conditioned media and cell lysate were loaded. (B) LGI1 immunoblot shows secretion of LGI1, LGI3 and LGI3-Myc secretion. (C) HA immunoblot shows LGI2B-HA and LGI4-HA secretion. (D) LGI3-Myc secretion is shown with 9E10 antisera (Myc). (E) Ponceau S staining is shown to demonstrate equal loading among conditioned media samples and among cell lysate samples.
Figure 5. Intracellular localization of LGI2B-HA and LGI4-HA. HA epitope tagged proteins are visualized with anti-HA primary antibody and Texas Red secondary antisera (red), transfected cells (green) are visualized by GFP expression plasmid, and DAPI is used to identify nuclei (blue). (A) 3T3 cells transfected with LGI2B HA plasmid; (B) L929 cells transfected with LGI2B HA plasmid; (C) 3T3 cells transfected with LGI4 HA plasmid and (D) L929 cells transfected with LGI4-HA plasmid.
Figure 6. Mutations in LGI1 inhibit secretion and alter stability. (A) Schematic representation of LGI1 mutants and position of mutations. (B) Normal exposure of Flag immunoblot with the indicated transfections. (C) Long exposure of conditioned media samples to reveal low levels of secretion of several of the mutants. (D) Ponceau S staining is shown to demonstrate that all conditioned media samples were loaded equally and that all cell lysate samples were loaded similar to each other. Conditioned media samples were equalized to each other on the basis of total protein concentration, as were cell lysate samples, and then equivalent volume proportions of conditioned media and cell lysate were loaded.
References
Ottman, R., Risch, N., Hauser, W.A., Pedley, T.A., Lee, J.H., Barker-Cummings, C., Lustenberger, A., Nagle, K.J., Lee, K.S., Scheuer, M.L. et al. (
Kalachikov, S., Evgrafov, O., Ross, B., Winawer, M., Barker-Cummings, C., Martinelli Boneschi, F., Choi, C., Morozov, P., Das, K., Teplitskaya, E. et al. (
Morante-Redolat, J.M., Gorostidi-Pagola, A., Piquer-Sirerol, S., Saenz, A., Poza, J.J., Galan, J., Gesk, S., Sarafidou, T., Mautner, V.F., Binelli, S. et al. (
Chernova, O.B., Somerville, R.P. and Cowell, J.K. (
Kunapuli, P., Chitta, K.S. and Cowell, J.K. (
Kunapuli, P., Kasyapa, C.S., Hawthorn, L. and Cowell, J.K. (
Bashaw, G.J. and Goodman, C.S. (
Brose, K., Bland, K.S., Wang, K.H., Arnott, D., Henzel, W., Goodman, C.S., Tessier-Lavigne, M. and Kidd, T. (
Wu, W., Wong, K., Chen, J., Jiang, Z., Dupuis, S., Wu, J.Y. and Rao, Y. (
Zhu, Y., Li, H., Zhou, L., Wu, J.Y. and Rao, Y. (
Scheel, H., Tomiuk, S. and Hofmann, K. (
Staub, E., Perez-Tur, J., Siebert, R., Nobile, C., Moschonas, N.K., Deloukas, P. and Hinzmann, B. (
Runkel, F., Michels, M. and Franz, T. (
Bisulli, F., Tinuper, P., Scudellaro, E., Naldi, I., Bagattin, A., Avoni, P., Michelucci, R. and Nobile, C. (
Ottman, R., Winawer, M.R., Kalachikov, S., Barker-Cummings, C., Gilliam, T.C., Pedley, T.A. and Hauser, W.A. (
Berkovic, S.F., Izzillo, P., McMahon, J.M., Harkin, L.A., McIntosh, A.M., Phillips, H.A., Briellmann, R.S., Wallace, R.H., Mazarib, A., Neufeld, M.Y. et al. (
Hedera, P., Abou-Khalil, B., Crunk, A.E., Taylor, K.A., Haines, J.L. and Sutcliffe, J.S. (
Michelucci, R., Poza, J.J., Sofia, V., de Feo, M.R., Binelli, S., Bisulli, F., Scudellaro, E., Simionati, B., Zimbello, R., D'Orsi, G. et al. (
Fertig, E., Lincoln, A., Martinuzzi, A., Mattson, R.H. and Hisama, F.M. (
Pizzuti, A., Flex, E., Di Bonaventura, C., Dottorini, T., Egeo, G., Manfredi, M., Dallapiccola, B. and Giallonardo, A.T. (
Gu, W., Brodtkorb, E. and Steinlein, O.K. (
Jossin, Y. and Goffinet, A.M. (
O'Brien, R.J., Xu, D., Petralia, R.S., Steward, O., Huganir, R.L. and Worley, P. (
Ramarao, M.K., Bianchetta, M.J., Lanken, J. and Cohen, J.B. (