-
PDF
- Split View
-
Views
-
Cite
Cite
Rose-Marie Vouimba, Gal Richter-Levin, Physiological Dissociation in Hippocampal Subregions in Response to Amygdala Stimulation, Cerebral Cortex, Volume 15, Issue 11, November 2005, Pages 1815–1821, https://doi.org/10.1093/cercor/bhi058
Close - Share Icon Share
Abstract
Previous studies indicated that the amygdala, when activated by emotional or electrical stimulation, modulates hippocampal-dependent memory processes and synaptic plasticity. Although the modulatory effect of the amygdala has often been generalized to the hippocampal formation, studies suggest that hippocampal subregions may display distinct functional profiles and may respond distinctively to amygdala activation. In this study we assessed the effect of basolateral amygdala (BLA) stimulation on long-term potentiation (LTP) — a synaptic model of memory — induced by a standard (sdTS) or a strong theta stimulation (sgTS) in the hippocampal dentate gyrus (DG) and CA1, in anesthetized rats. The modulatory stimulation was applied 30 s before or after the tetanus stimulation. Results show that while BLA activation impaired CA1 LTP induced with an sdTS, it enhanced LTP in the DG under both sdTS and sgTS conditions. These findings provide evidence for a differential amygdalar control of hippocampal memory subsystems, and may contribute to the understanding of the complexity of memory processes under stressful conditions.
Introduction
The relationship between stress and learning is complex. Human and animal studies that have assessed its effects on subsequent memory processing reported that stress may impair, not affect or enhance, memory performance. On the one hand, stressful experiences and/or elevated levels of the stress hormones corticosterone can induce lasting changes in microstructure and function of brain areas involved in memory. Exposure to a variety of acute stressors or corticosterone can impede hippocampal-dependent memory processes, such as acquisition (Oitzl and de Kloet, 1992; Kirschbaum et al., 1996; Roozendaal and McGaugh, 1997) or retrieval (Diamond and Rose, 1994; Diamond et al., 1996; de Quervain et al., 2000; Rashidy-Pour et al., 2004). In addition, acute stress can impair long-term potentiation (LTP) (Foy et al., 1987; Diamond and Rose, 1994; Maroun and Richter-Levin, 2003), a model of synaptic plasticity believed to underlie memory formation (Bliss and Collingridge, 1993, Malenka and Nicoll, 1999). On the other hand, exposure to acute stressful experience or stress hormones can either facilitate or leave unaffected hippocampal memory processes and synaptic plasticity in different hippocampal subregions (Servatius and Shors, 1994; Akirav et al, 2001; Shors, 2001; Domes et al., 2002; Cahill et al., 2003; Vouimba et al., 2004).
Studies suggest that the amygdala may mediate stress-related alterations of hippocampal memory processes and LTP (Akirav and Richter-Levin, 1999; Kim et al., 2001; McGaugh, 2002). Particularly, the basolateral amygdala complex (BLA) is believed to be essential for both stress-induced impairment and enhancement of hippocampal function (Liang et al., 1994; Akirav and Richter-Levin, 1999; Kim et al., 2001). For instance, BLA lesions and/or pharmacological manipulations disrupt memory-modulating processes initiated by systemic or intrahippocampal infusion of drugs (Roozendaal and McGaugh, 1996, 1997; Packard and Chen, 1999) and block the adverse effects of acute stress on hippocampal LTP and memory (Kim et al., 2001). Furthermore, electrical stimulation of the amygdala increases the magnitude of hippocampal dentate gyrus (DG) LTP (Ikegaya et al., 1995; Frey et al., 2001), and animals with amygdala damage have reduced DG LTP (Ikegaya et al., 1994). Akirav and Richter-Levin (1999, 2002) elaborated on these effects and reported that BLA activation could induce either an excitatory or an inhibitory effect on DG LTP, depending on the temporal profile of stimulation. Specifically, these authors showed that a priming stimulation of the BLA 30 s prior to application of strong theta stimulation (sgTS) to the perforant path (PP) enhanced LTP in the DG. In contrast, spaced stimulation of the BLA (1 h prior to sgTS) prevented LTP enhancement (Akirav and Richter-Levin, 1999). Most recently, Nakao et al. (2004) reported that the effects of amygdala activation on DG synaptic plasticity could range from LTP to LTD, depending on the degree and timing of neural activity of the BLA (Nakao et al., 2004).
The stress-induced alteration of hippocampal functioning has often been generalized to the hippocampal formation. However, studies indicated that hippocampal subregions display distinct functional profiles. For instance, the DG and CA1 have been shown to be involved in different aspects of spatial learning (Gilbert et al., 2001; Lee and Kesner, 2003), so these subregions could respond differentially to stress. In fact, studies have shown that the DG and CA1 display a distinct susceptibility to acute stress (Gerges et al., 2001; Maroun and Richter Levin, 2003; Vouimba et al., 2004). Namely, acute exposure to platform stress disrupts LTP in CA1 but not in the DG (Maroun and Richter-Levin, 2003; Vouimba et al., 2004). Furthermore, discriminatory avoidance learning, which by its nature evokes a substantial stress response, selectively inhibits LTP in the hippocampal CA1 region, while enhancing LTP in the DG (Izaki and Arita, 1996). Such dissociation in hippocampal subregions may be relevant to the understanding of the twofold effect of stress on hippocampal-dependent learning and memory.
The purpose of this study was to elaborate on the mechanisms underlying these observations and further to assess the effects of amygdala activation on LTP in the CA1 and DG subregions. Here we show that BLA activation impairs LTP in the CA1 subfield, while enhancing LTP in the DG.
Materials and Methods
Subjects
The experiments were performed using male Sprague–Dawley rats (Harlan Laboratories, Jerusalem, Israel) weighing 200–280 g. They were housed in Plexiglas cages (five rats per cage) and were maintained on a free-feeding regimen with a 12:12 h light/dark schedule. All electrophysiological testing was performed at least 1 week after their arrival, during the light phase of the cycle. During the course of all the experiments, body temperature was monitored and maintained at 37 ± 0.5°C using a regulated heating pad. The experiments were carried out in accordance with the guidelines of the University of Haifa Ethics and Animal Care Committee.
Electrophysiology
Surgery
Rats were anesthetized (40% urethane + 5% chloral hydrate, 0.5 ml/100 g, i.P.) and mounted on a stereotaxic frame (Stolting, Wood Dale, IL). The scalp was incised and retracted, and the head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr holes (∼2 mm diameter) were drilled in the skull for the placement of stimulating and recording electrodes. A reference electrode consisting of a 125 μm coated wire was affixed to the skull in the area overlapping the nasal sinus. A recording glass electrode (tip diameter, 2–5 μm; filled with 2 M NaCl) was stereotaxically positioned in the CA1 pyramidal cell layer [4.2 mm posterior to bregma (AP), 2.5 mm from midline (ML) and ∼2 mm dorsoventral (DV)] or in the DG granular cell layer (4.2 mm AP, 2.5 mm ML and ∼3.7 mm DV). Bipolar concentric stimulating electrodes (125 μm; Kopf, Tujunga, CA) were inserted in the ipsilateral BLA (3 mm AP, 5.2 mm ML and 7.6 mm DV) and either in the contralateral ventral hippocampal commisure (vHC: 2 mm AP, 1.5 mm ML and ∼3 mm DV) for activating field potentials in the CA1 or in the ipsilateral PP (8 mm AP, 4 mm ML and ∼3 mm DV) for activating field potentials in the DG. The dorsoventral location of the recording and stimulating electrodes was adjusted to maximize the amplitude of evoked field potentials.
Stimulating and Recording Procedures
CA1 and DG field potentials evoked by single pulses delivered to the vHC or PP, respectively (0.1 ms rectangular monophasic pulses) were amplified (×1000) by a WPI amplifier, displayed on an oscilloscope, digitized at 10 kHz (CED) and stored to disk for off-line analysis (Signal-2 software). Baseline responses were established by means of a stimulation intensity (50–150 μA) sufficient to elicit a response representing ∼25–30% of the maximal amplitude of the evoked field potentials.
LTP was assessed by measuring both the increase in the population spike amplitude (PS) and the slope of the excitatory postsynaptic potential (EPSP) component, for both the DG and CA1. However, in CA1, some recordings from which the slope was not measurable (because the population spike occurred early) were excluded from the EPSP slope analysis (n = 5).
Protocols
Baseline recording of evoked field potential was established for 30 min (1 pulse every 30 s) for all groups. In the Control sdTS group, baseline recording in CA1/DG was followed by application of a standard theta-like high-frequency stimulation (sdTS: one set of five trains, each train consisting of five pulses at 100 Hz, with an inter-train interval of 200 ms) to the vHC or PP. After sdTS stimulation, responses to test pulse stimuli were recorded every 30 s for 1 h. In the Control no sdTS group, after the 30 min baseline, responses were recorded for 1 h. In the Control BLA group, baseline recording was followed by BLA stimulation (1 V, 50 μs pulse duration, 10 trains of five pulses at 100 Hz; intertrain interval = 200 ms) followed again by 1 h of recording. In the experimental groups the baseline recording was followed by stimulation of the BLA either 30 s before (BLA prior sdTS) or 30 s after (BLA post sdTS) sdTS to the vHC or PP. Responses to low-frequency baseline pulses were then collected every 30 s for 1 h.
In another subset of animals, in which electrodes were placed in the vHC–CA1 or PP–DG pathways, a strong theta (three sets of 10 trains; each train consisted of 10 pulses at 100 Hz; intertrain interval = 200 ms; interset interval = 1 min) was given under Control sgTS (n = 11) and BLA prior sgTS (n = 13) conditions. This sgTS has been reported to induce, in the DG, LTP sensitive to BLA activation (Akirav and Richter-Levin, 1999, 2002).
Histology
After completion of the study, animals with a stimulating electrode in the BLA were placed under deep anesthesia and transcardially perfused with physiological saline, followed by 10% buffered formalin. Brains were post-fixed in formalin–saccharose 30% solution for at least 3 days, frozen and cut coronally into 80 μm sections on a sliding microtome. The sections were mounted on a gelatin-coated slide, and stained with cresyl violet for microscopic examination of the placements of the stimulating electrode in BLA.
Data Analysis
The amplitude of the PS and the slope of the EPSP were expressed as the mean percentage (± SEM) of the individual basal values of animals for each group. Group differences were analyzed by analysis of variance (ANOVA) and post hoc Fisher tests (Statview).
Results
Histology
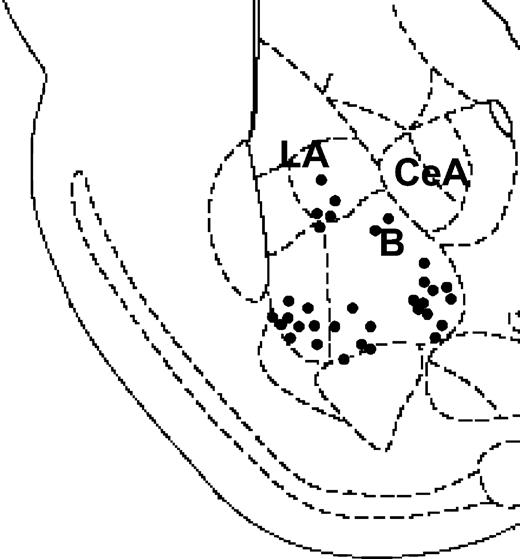
Figure 1 illustrates the location of stimulating site in BLA. Histological analysis revealed that 59 of the 70 rats recorded had a correct positioning of the electrode in the BLA. Only data from these subjects were used in the analysis.
A diagram depicting a coronal section of the rat brain (∼3.00 mm posterior to bregma) showing electrode placements in the BLA. Filled circles indicate locations for the groups ‘BLA prior’ and ‘BLA post’ for both CA1 and DG (n = 33; B, basal amygdala; La, lateral amygdala; CeA, central amygdala).
Effects of BLA Activation on Basal Synaptic Transmission and Neuronal Excitability in CA1 and the DG
The field potentials evoked in CA1 and the DG of the three control groups were stable throughout the 30 min of baseline recording [Figs 2 and 3; F(2,18) < 1 and F(2,19) < 1; ns]. Activation of the BLA increased excitability in the DG granular cells but not in CA1 pyramidal cells. A two-way ANOVA with repeated measures performed on DG responses revealed a significant increase with respect to both baseline and control no sdTS [group: F(1,11) = 15.65; P < 0.001; group × time: F(18,198) = 8.08; P < 0.001; see Figs 2a and 3a]. In addition, BLA activation did not affect baseline synaptic transmission in the vHC–CA1 and PP–DG pathways [Figs 2a and 3a; F(18,198) < 1 and F(18,180) < 1; ns].
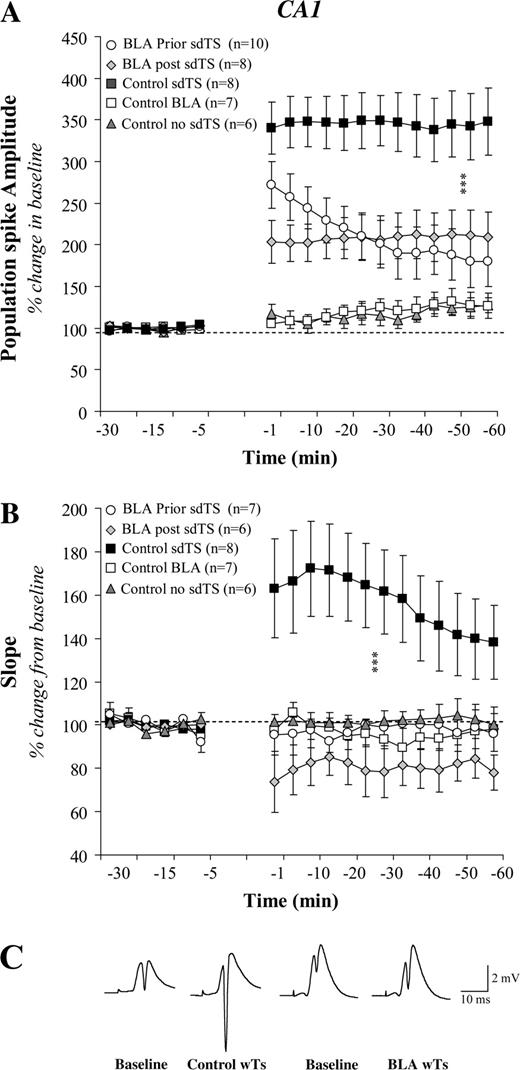
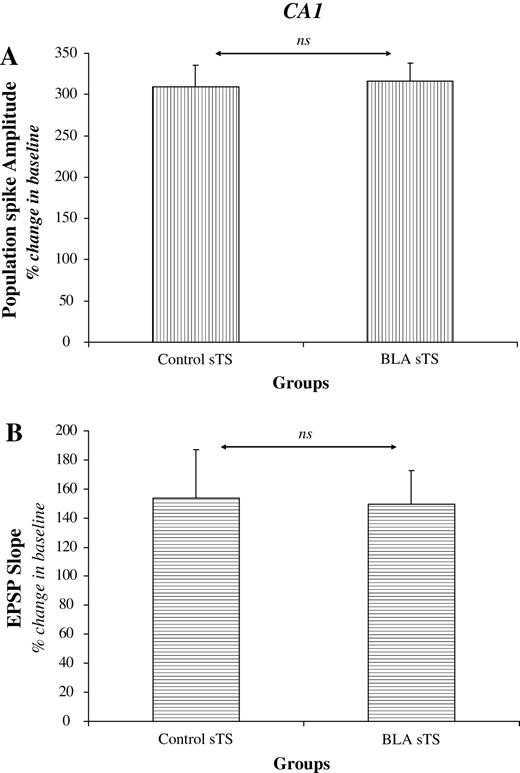
Effect of BLA activation on CA1 LTP induced by sdTS: mean (± SEM) percentage of baseline. BLA activation, 30 s before or after sdTS, impaired LTP of PS amplitude (A) and the slope of the EPSP component (B). No significant changes were observed in the baseline synaptic transmission. Representative analog traces recorded during baseline and following sdTS from an individual of the Control and BLA prior groups (C). Asterisks (***) indicate significant differences (P < 0.001) between control and other groups using post hoc Fisher tests.
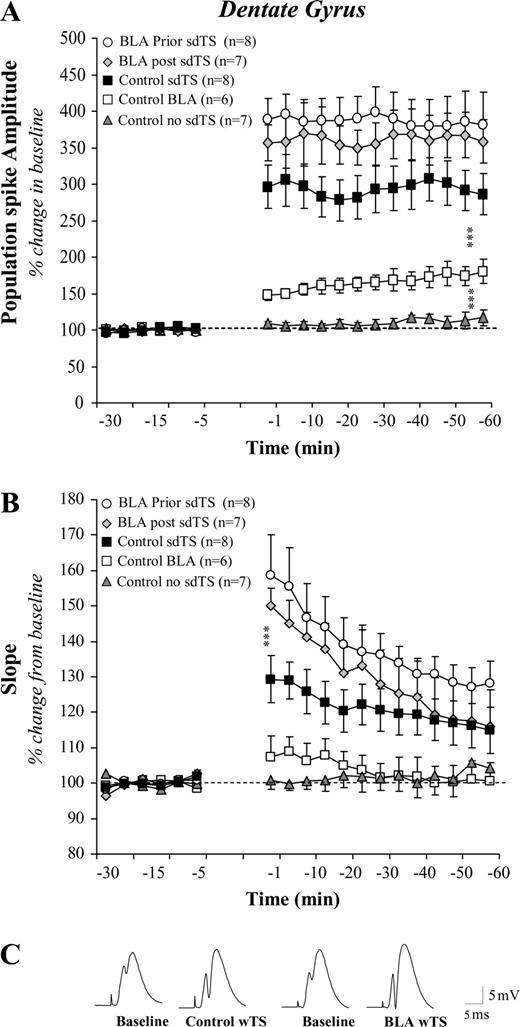
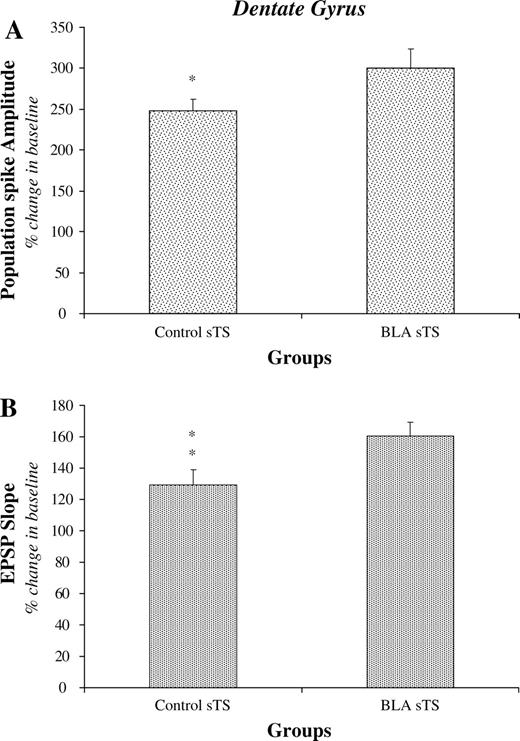
Effect of BLA activation on DG LTP induced by sdTS: Mean (± SEM) percentage of baseline. Activation of the BLA increased granular cell excitability. In addition, BLA activation, 30 s before or after sdTS enhanced PS amplitude (A) and the slope of the EPSP component (B). Representative analog traces recorded during baseline and following sdTS from an individual of the Control and BLA prior groups (C). Asterisks (***) indicate significant differences (P < 0.001) between control and other groups using post hoc Fisher tests.
Thus, BLA activation induced an increase in granular cell excitability but not in pyramidal cell excitability.
Effects of BLA Activation on LTP Induced by sdTS in CA1 and the DG
In control animals, sdTS applied to the vHC–CA1 or PP–DG pathways produced an increase in both the magnitude of the PS and the slope of the EPSP component. A two-way ANOVA analysis revealed a significant increase with respect to baseline for both CA1 and the DG (PSs amplitude: Ps < 0.0001; EPSPs slope: Ps < 0.0001; Figs 2 and 3).
Stimulating the BLA 30 s prior to application of sdTS to PP–DG enhanced the PS amplitude (Fig. 3a) as well as the slope of the EPSP (Fig. 3b). The same effect was observed if the BLA was stimulated 30 s after LTP induction. Statistical analyses indicated a significant difference between groups [PS: F(4,31) = 23.33; P < 0.0001; EPSP: F(4,31) = 6.14; P = 0.0009). Group comparisons showed that animals that received BLA stimulation before or after sdTS to the PP developed significantly more LTP than controls (all Ps < 0.001).
In CA1 a quite different effect was encountered: BLA activation 30 s prior to (BLA prior sdTS) or 30 s after (BLA post sdTS) the application of sdTS to the vHC impaired LTP in the vHC–CA1 pathway (Fig. 2a and 2b). ANOVAs revealed a significant difference between groups [PS: F(4,34) = 13.48; P < 0.0001; EPSP: F(4,29 = 6.64; P = 0.0006]. Animals that received BLA stimulation before or after sdTS presented a suppression of LTP, in contrast to control sdTS (all Ps < 0.0001). Comparing PS magnitude in BLA prior sdTS with that in BLA post sdTS revealed no significant difference (P > 0.05) except for the magnitude of short-term plasticity (PTP) occurring 1–5 min post-tetanus (P = 0.049). In contrast, there was a significant difference in EPSP slope levels between the BLA post sdTS and BLA prior sdTS groups (P = 0.0006). Thus, BLA activation differentially modulated hippocampal plasticity, in CA1 and the DG, induced by standard theta stimulation.
Effects of BLA Activation on LTP Induced by sgTS, in CA1 and the DG
As previously reported (Akirav and Richter-Levin, 1999, 2002), amygdala stimulation enhanced LTP in the DG also under sgTS condition (Fig. 4a,b). Statistical analyses revealed a significant difference between the Control and BLA prior groups for both the PS and the EPSP (Ps < 0.05).
Effects of BLA activation on DG LTP induced by sgTS (period from 51 to 60 min following theta stimulation). BLA modulation enhanced significantly both the size of the PS (A) and the slope of the EPSP (B). **P < 0.01; *P < 0.05.
In CA1, amygdala activation did not impair LTP induced with sgTS (Fig. 5a,b). Statistical analyses revealed no significant difference between the Control sgTS and BLA prior sgTS groups (P > 0.05 for both the PS and the EPSP).
Effects of BLA activation on CA1 LTP induced by sgTS (period from 51 to 60 min following theta stimulation). Strong TS induction protocol overcame the effect of BLA modulation on CA1 LTP. There were no significant differences in the magnitude of the PS (A) and EPSP (B) between the Control and BLA prior groups (ns = non-significant).
Discussion
We examined the effects of BLA activation on field potential responses and synaptic plasticity in two hippocampal areas: the PP input to the granule cells in the DG, and the vHC input to the CA1 pyramidal cells. Our results showed that stimulating the BLA 30 s prior to application of an sdTS or sgTS to the PP enhanced LTP in the DG. The same effect was obtained by activating the BLA 30 s after LTP induction. Conversely, activating the BLA before or after sdTS to the vHC impaired LTP in CA1. In addition, under the sgTS condition, amygdala-induced impairment of CA1 LTP was prevented.
Previous studies indicate that CA1 LTP induced with a standard tetanus (primed burst potentiation) was more sensitive to stress or drug modulation than LTP induced with a strong tetanus (Diamond et al., 1999; Mesches et al., 1999; Vouimba et al., 2003). Straube and Frey (2003) also reported that a strong LTP-induction protocol, compared with a weaker one, prevented drug impairment of late-LTP in the DG. Mechanistically, weak or standard tetanus was found to induce LTP via activation of calcium influx through the NMDA receptor channel complex. In contrast, strong tetanus induced an NMDA receptor-independent LTP, through activation of voltage-gated calcium channels (VGCCs) (Grover and Teyler, 1990). Thus, a possible mechanism for the differential result obtained in CA1 in the present study could be that strong and standard theta stimulation induced two different types of LTP in CA1, carrying differential sensitivities to amygdalar modulation.
In addition to intensity parameters, our experiments also varied the timing of BLA stimulation. Activation of BLA 30 s after LTP induction had a more profound effect on CA1 plasticity than stimulation applied 30 s before LTP induction. BLA stimulation applied after tetanization impaired both CA1 population spike PTP and LTP, while its activation prior to LTP induction altered only LTP. Finally, BLA stimulation suppressed EPSP slope LTP and showed a trend toward LTD when applied after tetanization of CA1. One possible explanation for the greater effect of post-tetanic BLA activation is an alteration in BLA sensitivity following LTP induction in the CA1. We have recently reported that tetanic stimulation applied to the vHC produced LTP also in the BLA (Vouimba et al., 2003). Thus, an increase in BLA neuronal plasticity may account for its subsequent effect on CA1 LTP.
Alternatively, this modulation of CA1 LTP displays characteristics reminiscent of behavioral results showing that memory consolidation of recent experiences can be altered, by various treatments, after a learning event (see Roozendaal, 2002; McGaugh, 2004). For example, compelling evidence indicates that post-training infusions of drugs and neurotransmitters into the amygdala can modulate the strength of memory consolidation for different hippocampal-dependent tasks (Castellano et al., 1989; Izquierdo et al., 1990; McGaugh, 2000; LaLumiere et al., 2003). Similarly, muscimol infusion into the amygdala impaired 24h retention of an inhibitory avoidance when administered immediately after training but had no effect when administered 30 min after training (Zanatta et al., 1997). Furthermore, Dopamine infused into the BLA immediately after training enhanced retention, whereas DA infused into the BLA 3 h after training of an inhibitory avoidance did not affect retention (LaLumiere et al., 2004). Additionally, Seidenbecher et al. (1997) reported that behavioral reinforcers (aversive and appetitive stimuli) were effective at modulating LTP when presented immediately after, but not before, tetanus-induced LTP.
In contrast to CA1, BLA-dependent enhancement of DG LTP was observed regardless of the tetanus parameters used in this study. However, variations in temporal and intensity parameters of BLA stimulation have been found to influence DG plasticity as well. Thus, spaced stimulation of the BLA was shown to suppress LTP in the DG (Akirav and Richter-Levin, 1999, 2002). Also, Nakao et al. (2004) showed that on a continuum of LTP/LTD induced by varying parameters of the tetanus applied to the PP, strong BLA activation favored LTP whereas weak BLA activation favored LTD. Thus, we cannot rule out time- and/or intensity-variable effects of BLA stimulation on DG LTP.
Another finding in this study was that BLA activation excited DG granule cells but not CA1 pyramidal cells. This concurs with the finding of Nakao et al. (2004) showing an increase in c-Fos-like immunoreactivity in the DG following BLA activation. The exact pathway by which BLA activity may affect DG excitability and plasticity is yet to be defined. Anatomical studies revealed that whereas the CA1 has direct connections with the BLA, there are no known direct efferents connecting the amygdala to the DG (Amaral, 1986; Chen et al., 1999; Pikkarainen et al., 1999). Lesion and electrophysiological studies have proposed the septo-hippocampal pathway and the entorhinal cortex as a relay between the BLA and DG (Frey et al., 2003; Yaniv et al., 2003; Nakao et al., 2004).
The differential effects of BLA activation on DG and CA1 synaptic plasticity echo those of acute stress exposure on LTP in these hippocampal subregions (Mesches et al., 1999; Maroun and Richter-Levin, 2003; Vouimba et al., 2003, 2004). Indeed, acute stress was reported to impede LTP in CA1 (Maroun and Richter-Levin, 2003) and to increase DG LTP (A. Kavushansky, R.M. Vouimba and G. Richter-Levin, unpublished data) or to have no effect on it (Vouimba et al., 2004). These data suggest that under stress BLA activation may favor treatment of information process by DG. In fact, behavioral studies indicate that CA1 and DG synapses play different functional roles in spatial learning and memory in a Morris water maze (Okada et al., 2003). The DG was proposed to support a spatial task, whereas CA1 supported a spatial temporal task, in a radial eight-arm maze (Xavier et al., 1999; Gilbert et al., 2001).
In sum, the data reported here suggest that the degree of amygdala engagement in hippocampal mnemonic processing may be dependent on the temporal relation between amygdala stimulation and hippocampal activation, as well as on the strength of hippocampal activation. Specifically, upon activation the BLA differentially affects information processing in the hippocampal subregions and modifies the relative involvement of these collaborating brain regions. The results underscore the need for an evaluation of the distinct contribution of hippocampal subfields to memory formation. Such region-dependent alterations may affect the dynamic interactions in hippocampal networks leading to differential consolidation processes. This approach might also benefit us with better understanding of the effects of emotional arousal and amygdalar activation on hippocampal functioning and memory.
This research was supported by The Israel Science Foundation — The Charles H. Revson Foundation (no. 582/00-1 to G.R.-L.). The authors thank Dr Dan Yaniv for helpful comments on the manuscript.
References
Akirav I, Richter-Levin G (
Akirav I, Richter-Levin G (
Akirav I, Sandi C, Richter-Levin G (
Amaral DG (
Bliss TV, Collingridge GL (
Cahill L, McGaugh JL (
Cahill L, Gorski L, Le K (
Castellano C, Brioni JD, Nagahara AH, McGaugh JL (
Chen GQ, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A (
de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C (
Diamond D, Rose GM (
Diamond DM, Fleshner M, Ingersoll N, Rose GM (
Diamond DM, Fleshner M, Rose GM (
Domes G, Heinrichs M, Reichwald U, Hautzinger M (
Foy MR, Stanton ME, Levine S, Thompson RF (
Frey S, Bergado-Rosado J, Seindenbecher T, Pape HC, Frey JU (
Frey S, Bergado JA, Frey JU (
Gerges NZ, Stringer JL, Alkadhi KA (
Gilbert PE, Kesner RP, Lee I (
Grover LM, Teyler TJ (
Ikegaya Y, Saito H, Abe K (
Ikegaya Y, Saito H, Abe K (
Izaki Y, Arita J (
Izquierdo I, Da Cunha C, Huang CH, Walz R, Wolfman C, Medina JH (
Kim JJ, Lee HJ, Han JS, Packard MG (
Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH (
LaLumiere RT, Buen TV, McGaugh JL (
LaLumiere RT, Nguyen LT, McGaugh JL (
Lee I, Kesner RP (
Liang KC, Hon W, Davis M (
Maroun M, Richter-Levin G (
McGaugh JL (
McGaugh JL (
Mesches MH, Fleshner M, Heman KL, Rose GM, Diamond DM (
Nakao K, Matsuyama K, Matsuki N, Ikegaya Y (
Oitzl MS, de Kloet ER (
Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S (
Packard MG, Chen SA (
Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A (
Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y (
Roozendaal B (
Roozendaal B, McGaugh JL (
Roozendaal B, McGaugh JL (
Seidenbecher T, Reymann KG, Balschun D (
Servatius RJ, Shors TJ (
Shors TJ (
Straube T, Frey JU (
Vouimba RM, Muñoz C, Diamond DM (
Vouimba RM, Yaniv D, Diamond D, Richter-Levin G (
Xavier GF, Oliveira-Filho FJ, Santos AM (
Yaniv D, Vouimba RM, Diamond DM, Richter-Levin G (