-
PDF
- Split View
-
Views
-
Cite
Cite
Silke Patz, Jochen Grabert, Thorsten Gorba, Marcus J. Wirth, Petra Wahle, Parvalbumin Expression in Visual Cortical Interneurons Depends on Neuronal Activity and TrkB Ligands during an Early Period of Postnatal Development, Cerebral Cortex, Volume 14, Issue 3, March 2004, Pages 342–351, https://doi.org/10.1093/cercor/bhg132
Close - Share Icon Share
Abstract
The differentiation of cortical interneurons is controlled by environmental factors. Here, we describe the role of activity and neurotrophins in regulating parvalbumin (PARV) expression using organotypic cultures (OTC) of rat visual cortex as model system. In OTC, PARV expression was dramatically delayed. The organotypic proportion of ∼6% PARV neurons was not established before 50–70 DIV, whereas in vivo all neurons are present until P20. Thalamic afferents increased cortical PARV mRNA in OTC, but not to the age-matched in vivo level. During the first 10 DIV, BDNF and NT-4 accelerated PARV mRNA expression in a Trk receptor and MEK2 dependent manner. The BDNF action required PI3 kinase signalling. PARV expression required activity. The proportion of neurons which managed to up-regulate PARV was inversely related to the duration of early transient periods of activity deprivation. Long-term activity-deprived OTC completely failed to up-regulate PARV mRNA. Both TrkB ligands failed to promote PARV expression in activity-deprived OTC. However, a few basket and chandelier neurons were observed, suggesting that the development of class-specific morphological features is activity-independent. Once established, PARV expression became resistant to late-onset activity deprivation. In conclusion, PARV expression depended on activity and TrkB ligands which appear to prime the PARV expression already before its developmental onset.
Introduction
In the mammalian cortex the main source of inhibition is provided by GABAergic interneurons. Besides neuropeptides serving as modulators of excitation (Klapstein and Colmers, 1997; Baraban, 1998), the calcium-binding proteins parvalbumin (PARV; Heizmann, 1984; Berchtold and Means, 1985), calretinin and calbindin are class-defining markers which also correlate with electrophysiological properties (Baimbridge et al., 1992; Gonchar and Burkhalter, 1997). They buffer intracellular free calcium during excitation (Dreessen et al., 1996), are neuroprotective (Van Den Bosch et al., 2002) and important for cellular function (Caillard et al., 2000; Vreugdenhil et al., 2002). PARV is considered essential for fast-spiking basket and chandelier cells which deliver inhibition to pyramidal cell somata and axonal initial segments, respectively (Fairen et al., 1994; Kawaguchi and Kubota, 1993, 1997) and PARV is present in the recently identified multipolar bursting neurons (Blatow et al., 2003). Thus, the acquisition of PARV expression could be of vital importance for the development of sufficient levels of cortical inhibition and even for cognition, since cortical PARV deficits are reported in schizophrenia (Zhang and Roberts, 2002; Paul Blum and Mann, 2002).
In rat visual cortex, PARV neurons appear during the second postnatal week (Alcantara et al., 1993; DeLecea et al., 1995). The neurons concurrently down-regulate calbindin and neuropeptide expression (Alcantara et al., 1996a; Cauli et al., 1997). Since PARV expression seems not inherited by lineage (Mione et al., 1994), several studies have already focused on environmental factors governing the onset and maintenance of PARV expression. In frontal cortex, dopaminergic afferents accelerate PARV expression (Porter et al., 1999; Ross and Porter, 2002). Rat visual cortex, however, is only sparsely innervated by dopaminergic afferents (Papadopoulos et al., 1989). Here, visual input influences PARV expression (Cellerino et al., 1992; Carder et al., 1996). Upon silencing the input from one eye, PARV expression declines exclusively in the binocular portion of the contralateral cortex; however, this effect is not seen after binocular deprivation or dark rearing (Cellerino et al., 1992). Lesioning thalamocortical afferents delays and lesioning basal forebrain afferents accelerates development of PARV structures in barrel cortex (Alcantara et al., 1996b). In parietal cortex cultures, the full set of PARV neurons develops only when explants were made after postnatal day 7, suggesting that PARV expression depends on cortex-extrinsic factors (Vogt Weisenhorn et al., 1998). The overexpression of BDNF in vivo accelerates interneuronal maturation as revealed by a faster appearance of PARV synapses (Huang et al., 1999). PARV neurons respond to neurotrophins because the neuropeptide expression in PARV neurons is regulated by trkB ligands (Wahle et al., 2000), since many neurons express trkB receptors (Cellerino et al., 1996; Gorba and Wahle, 1999). These results suggested a variety of environmental factors influencing developmental PARV expression.
In order to dissect the role of activity, specific afferents and neurotrophins we employed organotypic cultures (OTC) made from newborn cortex. We report here, first, that PARV expression in OTC is dramatically delayed and it is only slightly accelerated by thalamic afferents. Secondly, neuronal activity as a master regulator is required already before the developmental onset of PARV expression and, thirdly, TrkB signalling promotes PARV expression only during a discrete time window early in development.
Materials and Methods
Organotypic Cultures
Roller-tube OTC were prepared as described (Wirth et al., 1998; Wahle et al., 2000). In brief, visual cortex and posterior thalamus including lateral geniculate nucleus were explanted from pigmented rats on postnatal day 0 (P0, day of birth). Tissue blocks were cut into 350 µm thick coronal slices using a McIllwain chopper. The cultures were arranged as cortex monocultures or thalamocortical or corticocortical cocultures. Cultures were fed three times a week with semiartificial medium containing 25% horse serum, 25% Hank’s balanced salt solution, 50% Eagle’s basal medium, 1 mM l-glutamine (all from Life Technologies, Karlsruhe, Germany) and 0.65% d-glucose (Merck, Germany). To block spontaneous synaptically generated action potential activity (Klostermann and Wahle, 1999), 10 mM MgSO4 was supplemented to the medium (Wirth et al., 1998) at the day of preparation for the times indicated. In the figures, spontaneously active OTC were indexed as ‘+’ (plus) and activity-deprived OTC were indexed as ‘–’ (minus). To terminate the activity deprivation, cultures were transferred to fresh medium with 2 mM Mg2+. Neurotrophins (BDNF, NT-4, NT-3; all from Tebu, Frankfurt, Germany) were applied at 20 ng/ml medium every second day for the time periods indicated in the Results section or in figure legends. Pharmaceuticals were applied for the times indicated: K252a, 40 nM (Calbiochem, Bad Soden, Germany); PD98059, 30 µM (New England Biolabs, Beverly, USA); U0126, 10 nM (New England Biolabs, Beverly, USA); LY294002, 30 nM (Sigma, St Louis, MO).
Generation of cDNA Libraries and PCR
The mRNA was prepared from saline-perfused rat visual cortex areas 17 and 18, and two to four batches of five pooled monocultures at the ages or experimental conditions indicated in the figures using a Dynabead mRNA Direct Kit (Dynal, Hamburg, Germany). cDNA libraries were synthesized with Sensiscript reverse transcriptase (20 U/µl; Qiagen, Hilden, Germany) at 37°C for 60 min. PCR was preformed in a total volumen of 50 µl with Taq DNA polymerase (0.5 U/µl; Qiagen). The amplified region in base pairs for the detection of PARV mRNA expression was the same as the 240 bp cRNA riboprobe used for in situ hybridization (140–380 bp; Berchtold and Means, 1985; Berchtold, 1987). As standard, glucose-6-phosphate dehydrogenase (G6PDH, bases 2112–2272; Ho et al., 1988) was chosen, because it is constitutively expressed throughout development and lacks induction by activity (Inokuchi et al., 1996). PCR conditions were kept within the linear range determined for each product.
In situ Hybridization
Complementary to PCR, in situ hybridization was employed to analyze distribution and quantify percentages of PARV mRNA expressing neurons. Hybridization was performed on free-floating sections and OTC at 50°C with DIG-UTP labeled riboprobes transcribed from a 240 bp cDNA encoding rat PARV (kindly provided by Dr M.W. Berchtold; Berchtold and Means, 1985) followed by stringent washes (Wahle, 2002). As a negative control, PARV cRNA probes transcribed in sense orientation and hybridized to OTCs remained negative.
Immunohistochemistry
Immunohistochemistry was performed to analyze neuronal morphology. OTC were rinsed in 100 mM phosphate buffer pH 7.4 and fixed with cold 4% paraformaldehyde in phosphate buffer for 45 min. In vivo material was obtained during earlier studies. OTC and sections were washed three times in phosphate buffered saline (100 mM PBS, 10 min), incubated in 0.5% Triton X-100 in PBS for 30 min, rinsed in TBS and blocked for 1 h in 1% bovine serum albumin and 1% normal goat serum (Dakopatts) in TBS. The primary rabbit antiserum against PARV (1:1000; Swant, Bellinzona, Switzerland) was incubated overnight at 11°C. Biotin-conjugated secondary diluted 1:300 in blocking solution was incubated for 3 h, followed by a 2 h incubation in avidin–biotin–horseradish peroxidase complex (both reagents from Dakopatts, Hamburg, Germany). Peroxidase reactivity was developed with 0.02% 3,3′-diaminobenzidine and 0.002% H2O2 in 50 mM Tris buffer pH 7.4 for 10 min. Sections and OTC were dehydrated, cleared and coverslipped with DEPEX“.
Analysis
To assess the hybridizations, we used the counting strategy described previously (Wirth et al., 1998; Wahle et al., 2000; Patz et al., 2003). Briefly, the number of PARV mRNA expressing neurons was plotted with an Eutectics Neuron Tracing system (400× magnification). Neurons were counted as positive if the alkaline phosphatase staining showed at least a ring of blue reaction product around the nucleus. After thionin-counterstaining, the total number of neurons was determined per mm2 by counting neuronal nuclei (at 1000× magnification) in at least four different positions of every culture. The number of PARV neurons was then expressed as a percentage of total neuronal number. The number of OTC analyzed is given in Tables 1 and 2. Statistical analysis of cell counts was performed with the Mann–Whitney U-test.
To quantify the PARV mRNA expression, PCR gels were scanned and band intensities were densitometrically determined (Eagle Eye System“, Stratagene) and normalized to G6PDH expression. The normalized levels of PARV mRNA were then expressed relative to the normalized values of the experimental control conditions indicated in the Results and in the figures, which were set to 1. For the developmental profile (Fig. 1), the normalized expression at postnatal day 2 (P2) or DIV 2+ or DIV 2–, resp., was set to 1 in order to reveal the developmental increase over P2 in vivo and 2 DIV, resp., in OTC. The reason for choosing P2/DIV2 as reference was that PARV mRNA could not be reliably amplified before the second postnatal day. The mRNA expression was determined with a total of 8–16 PCRs run with two to four independent cDNA libraries (see figure legends). The graphs represent the mean with SEM. Statistical analysis was performed with the Mann–Whitney U-test.
The immunostained material delivered the morphological data. Representative PARV-ir neurons were reconstructed with a camera lucida (1000× magnification) and neuron types were classified by axonal morphology. Primary dendrites and somatic outlines (Camera Lucida drawings at 1000× magnification) were obtained from 150–350 PARV-ir neurons per experimental condition sampled in perpendicular stripes from layer I to white matter. Drawings of somatic outlines were digitized and somatic area was determined. Statistical analysis was performed with the nonparametric Mann–Whitney U-test.
Results
Developmental Expression of PARV mRNA
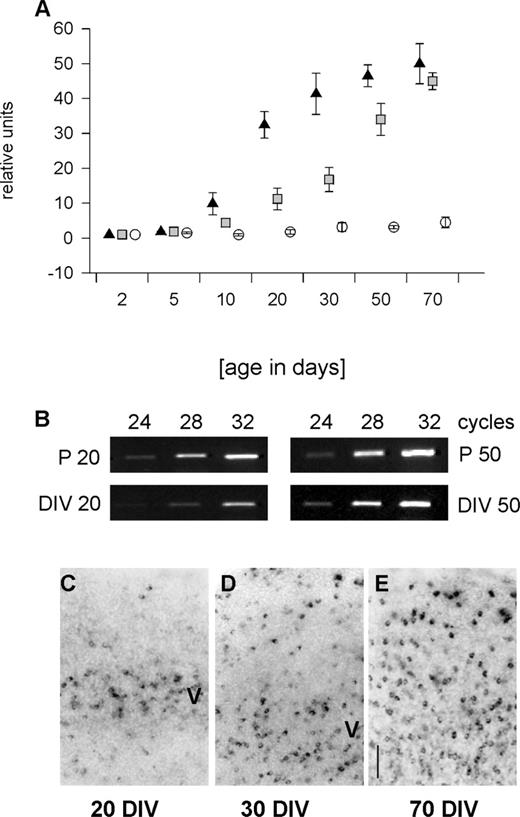
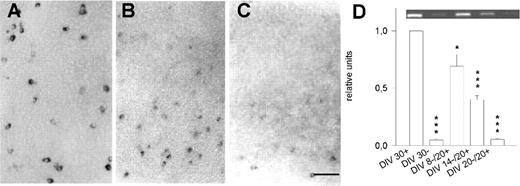
RT-PCR revealed that PARV mRNA in vivo was extremely low at birth (Fig. 1A). From P5 onwards a steep increase resulted in peak levels at P30–50. This developmental profile basically confirms earlier studies (Alcantara et al., 1993; DeLecea et al., 1995). In contrast, the time course in OTC was dramatically delayed (Fig. 1A). From 10 DIV onwards, the expression increased slowly. At 20 DIV, the expression was far below the age-matched in vivo level (Fig. 1B). At 50 DIV, OTC displayed PARV mRNA levels close to in vivo (Fig. 1B). An organoidentical expression level was reached at 70 DIV (Fig. 1A). This indicates a massive delay of PARV expression in OTC, although the neurons eventually manage to express the adult in vivo level.
Accordingly, the appearance of PARV mRNA expressing neurons was delayed (Fig. 1C–E and Table 1). PARV neurons were not detected in OTC younger than 10 DIV. By 15 DIV, ∼2% of the neurons expressed PARV mRNA (Table 1). At 20 DIV, neurons were present in layer V (Fig. 1C) as reported for parietal and frontal cortex OTC (Vogt Weisenhorn et al., 1998; Porter et al., 1999). Numbers had increased at 30 DIV and PARV neurons appeared in supragranular layers (Fig. 1D and Table 1). Until 70 DIV, ∼6% PARV neurons were found (Fig. 1E and Table 1) which is similar in proportion to in vivo, although in vivo all neurons are present by P20. This argues for a protracted inside first-outside last development.
The Role of Afferent Innervation
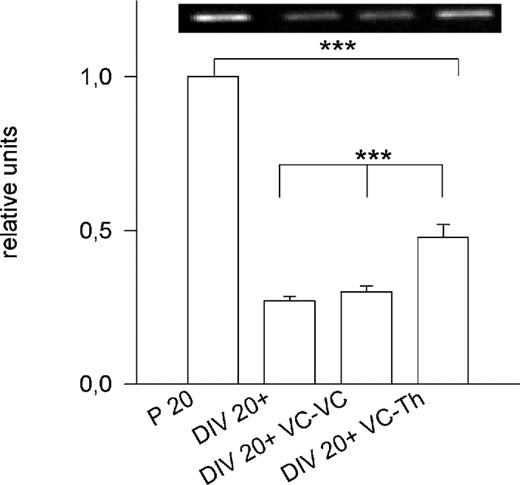
We next tested whether specific afferents accelerate PARV expression using corticocortical and corticothalamic cocultures. The reciprocal innervation forms until 12–14 DIV (Bolz et al., 1990). Compared to P20, the expression in 20 DIV OTC was much lower (Figs 1B and 2). Expression in corticocortical cocultures at 20 DIV was the same as in monocultures (P = 0.3). In contrast, thalamic afferents slightly accelerated cortical PARV expression: at 20 DIV, it was significantly higher than in monocultures (P < 0.001), although it remained significantly lower than the age-matched in vivo level (P < 0.001; Fig. 2).
Thus, neurons in OTC failed to time the onset of expression, suggesting that the accelerated PARV expression observed in vivo is driven by factors not conserved in OTC. Thalamic afferents do increase cortical PARV expression, but they failed to elicit the in vivo level. We next analyzed the role of neuronal activity and neurotrophins for PARV expression.
The Onset of PARV Expression in OTC depends on an Early Period of Activity
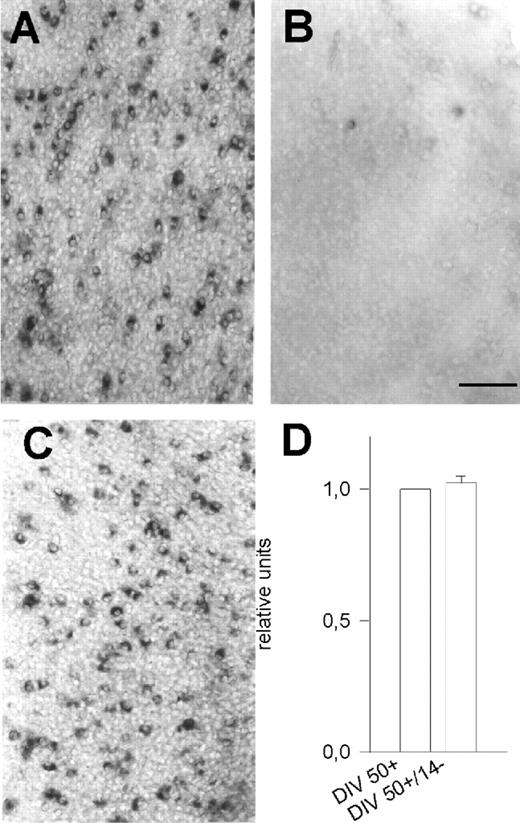
RT-PCR revealed that the PARV mRNA remained extremely low in OTC chronically deprived of neuronal activity (Fig. 1A). In situ hybridization in activity-deprived OTC revealed only 0.02–0.04% PARV mRNA expressing neurons at ages up to 70 DIV (Table 1 and Fig. 3, cf. A and B). This suggests that PARV expression is permanently suppressed in the absence of activity and no delayed up-regulation occured. By contrast, late-onset activity deprivation failed to down-regulate PARV. When 50 DIV spontaneously active OTC were activity-deprived for another 2 weeks, PARV mRNA expressing neurons remained present (Fig. 3C), and PCR revealed that the mRNA did not decline (Fig. 3D). This suggests, that once the PARV expression has been initiated, it becomes constitutive.
The percentage of PARV neurons appearing in OTC was dependent on an early period of activity. The longer this period was before the onset of activity deprivation (e.g. 0–5 DIV, 0–20 or 0–30 DIV; see Table 2), the more neurons were present. For instance, OTC were cultured in normal medium until 30 DIV and by that point many neurons had commenced PARV mRNA expression. OTC were then activity-deprived for another 20 DIV. However, in these 50 DIV OTC, we found 4.4 ± 0.8% PARV neurons, a value not very different from the 4.5 ± 1.1% observed in spontaneously active 30 DIV OTC (Table 1). Percentages always remained at the level present at the onset of deprivation without further increase to the percentages observed in age-matched active OTC.
Next, we tested whether the neurons recover PARV expression after an early transient period of activity deprivation. We knew from electrophysical recordings that returning activity-deprived OTC to normal medium leads to an immediate recovery of action potential activity and within 3–5 days the OTC establish de novo a balanced pattern of excitation and inhibition (Gorba et al., 1999). Indeed, the proportion of neurons which manage to up-regulate PARV was inversely related to the duration of an early transient period of activity deprivation (Table 2 and Fig. 4). Deprivation from 0–8 DIV followed by 20 DIV recovery in normal medium resulted in 2.5 ± 1.1% PARV mRNA expressing neurons (Fig. 4A and Table 2). Deprivation from 0–14 DIV followed by a 20 DIV recovery resulted in only 0.6 ± 0.2% PARV mRNA expressing neurons (Fig. 4B). A 0–30 DIV deprivation followed by 20 DIV recovery yielded only 0.03 ± 0.005% PARV mRNA expressing neurons (Fig. 4C), which was not greatly different from values obtained in chronically deprived OTC. This was confirmed by PCR (Fig. 4D).
Taken together, neuronal activity plays a pivotal role. For the up-regulation of PARV expression, putative PARV neurons essentially require neuronal activity already before the normal onset of expression. Accordingly, the earlier OTC are allowed to recover from activity deprivation, the more neurons manage to commence PARV mRNA expression. Once established, however, PARV expression becomes constitutive and is no longer activity-dependent.
The Role of Neurotrophins
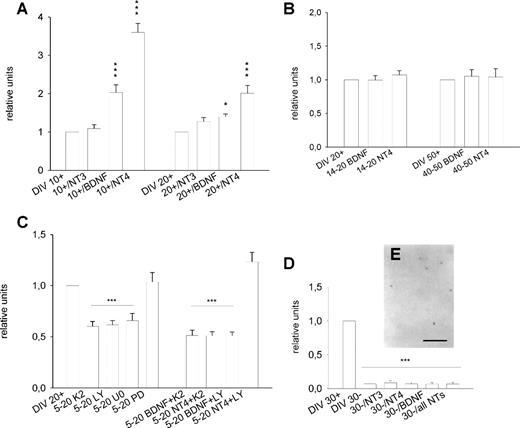
The known relation of neurotrophins and neurochemical maturation of interneurons led us to ask whether PARV expression can be accelerated by TrkB ligands. Indeed, BDNF and NT-4, but not NT-3, strongly promoted PARV mRNA expression when supplemented every second day from 0–10 DIV to spontaneously active OTC (Fig. 5A). However, when the factors were supplemented every second day from 0 to 20 DIV, the enhancing effect of BDNF was almost gone and that of NT-4 was much smaller (Fig. 5A).
In vivo, the increase of PARV expression concurrs with the rise of BDNF expression after eye opening (Schoups et al., 1995). The postnatal BDNF mRNA peak reported in vivo is missing in OTC (Gorba et al., 1999). Therefore, OTC were exposed to BDNF and NT-4 from 14 to 20 DIV and from 40 to 50 DIV. However, the factors entirely failed to promote PARV mRNA expression to the ‘adult’ levels present in 70 DIV OTC in both time windows (Fig. 5B). We also supplemented BDNF to spontaneously active OTC from 15 to 30 DIV and at 30 DIV we observed 4.3 ± 0.6% PARV neurons. This was not very different from the 4.5 ± 1.1% labeled neurons observed in age-matched untreated OTC and still significantly lower than the percentages observed at 70 DIV (Mann–Withney U-test, P < 0.01). It confirmed the declining effectiveness of BDNF with age. The results suggest that exogenous TrkB ligands accelerate PARV mRNA expression, but their action is limited to an early period in development.
Neurotrophins activate Trk receptors and various signalling pathways. PARV mRNA expression at 20 DIV was indeed reduced in the presence of the Trk inhibitor K252a (supplemented daily from 5 to 20 DIV), suggesting that endogenous neurotrophins drive the expression via the TrkB receptor (Fig. 5C). Concurrent inhibition of CamKII can be excluded, because this enzyme is only found in pyramidal cells (Sik et al., 1998). Further, PARV expression was reduced in the presence of the PI3 kinase inhibitor LY294002 and the MEK1/2 inhibitor U0126, but remained unchanged in the presence of the MEK1 inhibitor PD98059 (Fig. 5C) indicating that two alternative signalling pathways, either PI3 kinase or MEK2, are required to promote PARV transcription. Exogenous BDNF and NT-4 failed to promote PARV mRNA expression in the presence of K252a. BDNF also failed in OTC treated with LY294002 and thus depended on PI3 kinase signalling (for a review, see Heumann, 1994). In contrast, NT-4 promoted PARV expression in in the presence of LY294002, and thus acted PI3 kinase-independent (Fig. 5C).
NT-4 is known to substitute for the lack of neuronal activity in the case of neuropeptide Y (Wirth et al., 1998) and glutamic acid decarboxylase expression (Patz et al., 2003). Therefore, activity-deprived OTC were supplemented every second day with NT-4, BDNF, and NT-3, or a cocktail of all three factors until analysis at 30 DIV. However, PCR revealed that the factors and the cocktail failed to promote PARV expression in activity-deprived OTC (Fig. 5D). We found only very few PARV mRNA expressing neurons in 30 DIV OTC supplemented for instance with NT-4 (Fig. 5E). For each of the three neurotrophins, percentages remained at the level of age-matched untreated activity-deprived OTC (a mean of 0.03 ± 0.008%; n = 21; OTC treated with BDNF, NT-4 or NT-3 were pooled).
Taken together, TrkB ligands promote PARV expression early in development in a TrkB/MEK2/PI3 kinase-dependent manner, but they lose effectiveness with age. Further, the neurotrophin action requires the presence of neuronal activity.
The Morphological Differentiation of PARV-ir Neuron Types is Activity-independent
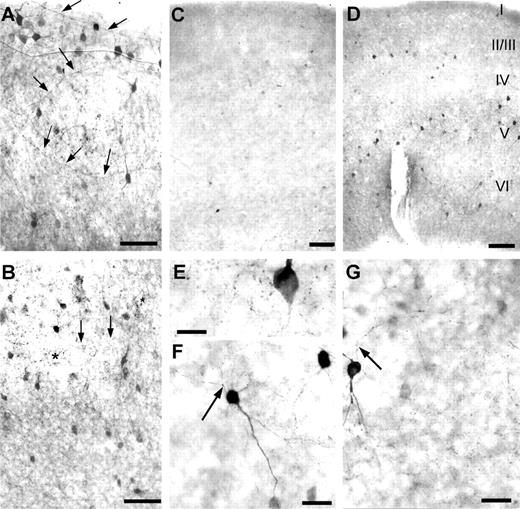
The slow maturation of PARV-ir structures was also observed with immunohistochemistry. Eventually however, 50 DIV OTC displayed a density and distribution of PARV-ir neurons and neuropil staining which is qualitatively similar to the known in vivo pattern (Fig. 6A, upper layers; B, lower layers). In particular, horizontal axons were observed in middle layers (III/IV/V), together with a typical feature of parvalbuminergic innervation which are the perisomatic boutons of PARV-ir basket cells terminating on non-parvalbuminergic pyramidal cell somata (Fig. 6B).
As expected, very few PARV-ir neurons were present in activity-deprived OTC (Fig. 6C). Appreciable numbers of neurons and neuropil appeared during recovery from early short-term deprivation (Fig. 6D). In activity-deprived OTC, a faintly labeled network of axonal varicosities and perisomatic boutons was present in the vicinity of PARV-ir neurons suggesting the development of interneuronal synaptic connections (Fig. 6E). Against this faint neuropil staining, the axonal patterns were detectable (Fig. 6F,G).
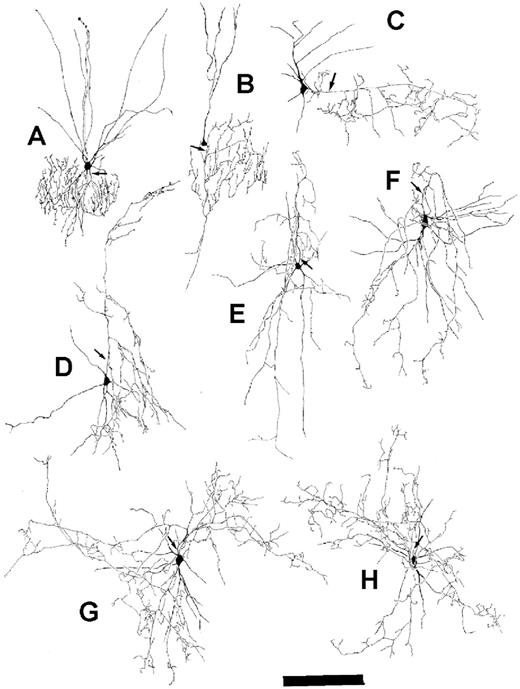
The morphology of PARV-ir neurons from active OTC (Fig. 7A,E) was qualitatively compared to activity-deprived OTC (Fig. 7B–D,F–H). In both experimental conditions we found chandelier cells and basket cells. Chandelier cell axons had local axon terminal fields. Typically, collaterals ended in vertically oriented ‘string-of-pearl’ segments close to the basal pole of pyramidal cell somata. The neurons had poorly branching dendrites reaching layer I. Although cell (B) differentiated under chronic activity deprivation, its axonal field was similar to cell (A) in terms of size and arborization. A small basket neuron of layer II/III reconstructed from an activity-deprived OTC (C) displayed an axon that extends horizontally beyond the dendritic field forming several ascending and descending collaterals within supragranular layers. Another cell type observed in layers III–V of spontaneously active (E) as well as deprived OTC (D,F) were multipolar basket cells with columnar arcade-type axons mostly arising from the upper cell pole. Large basket cells (G,H; both cells reconstructed from activity-deprived OTC) had long collaterals projecting horizontally in middle layers forming perisomatic endings on infragranular pyramidal cell somata. The morphology of these PARV-ir neuron types matched those obtained with biocytin fillings (although the latter were completely stained; see Klostermann and Wahle, 1999). These results suggest, that PARV neurons develop and maintain their classical morphological features in the absence of activity.
Somatic Growth of PARV Neurons is Affected by Activity Deprivation
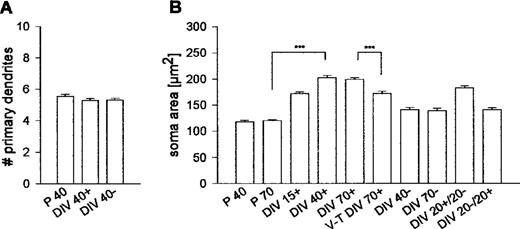
We determined the number of primary dendrites in ∼150 neurons each from spontaneously active and activity-deprived OTC (Fig. 8A). Values were not significantly different (Mann–Witney U-test, P = 0.7752); furthermore, neither sample was different from PARV neurons in vivo (determined at P30 in area 17; activity-deprived OTC versus in vivo, P = 0.2101; active OTC versus in vivo, P = 0.1043). The number of primary dendrites thus was not influenced by activity.
However, somatic growth was affected (Fig. 8B). In vivo, PARV neurons had aquired mature size variation at P40 without further growth at P70. In contrast, PARV neurons in active OTC grew hypertrophic and already at 15 DIV had larger somata than mature neurons in vivo. The final size variation was reached at 40 DIV. Strikingly, neurons sampled from 70 DIV thalamocortical cocultures were highly significantly smaller than neurons in 70 DIV monocultures (P < 0.001).
By contrast, neurons in 40 and 70 DIV activity-deprived OTC were highly significantly smaller (DIV 40– versus DIV 40+, P < 0.001) although the average size did not fall below in vivo values. Late-onset deprivation failed to reduce the soma size. The 20 DIV recovery after 20 DIV activity deprivation did not evoke somatic growth (DIV 20–/20+ versus DIV 20+/20– or versus DIV 40+, both P < 0.001).
This suggests that somatic growth is influenced by activity and by the presence of afferents. Further, the results from the late-onset deprivation and the recovery condition argue for the presence of a time window (earlier than 20 days) during which volume regulation of PARV cells has to proceed.
Discussion
PARV Expression depends on Neuronal Activity
Previous studies already described the delay and partial failure of PARV expression in cortex cultures (Porter et al., 1999; Ross and Porter, 2002), or the appearance of supragranular PARV neurons only in OTC explanted after P7 (Vogt Weisenhorn et al., 1998). However, aged OTC have not been analyzed in these studies. Our results now reveal that cortex-intrinsic mechanisms are sufficient for up-regulating PARV mRNA and cell numbers to the in vivo level albeit with a considerable delay. The sudden onset of PARV expression during the third postnatal week in vivo must depend on accelerating ‘factors’, e.g. afferent innervation. The physical presence of thalamic afferents did increase PARV mRNA, likely due to the fact that thalamic fibers synapse with PARV neurons. Further, thalamic afferents down-regulate cortical expression of leukemia inhibitory factor, and this evokes a down-regulation of NPY expression specifically in PARV neurons (Wahle et al., 2000). It remains to be tested whether LIF prevents the timely up-regulation of PARV expression. However, thalamic afferents failed to evoke the full expression level. In contrast, the absence of basal forebrain afferents in our OTC had apparently no stimulating effect on PARV expression, in contrast to Alcantara et al. (1996b).
Preventing action potential activity completely prevented PARV expression. Most interesting, PARV expression level and neuron numbers were positively correlated with the initial time period of activity: the longer young OTC experience activity, the more PARV neurons appear. In turn, PARV expression level and neuron numbers were inversely related to the time period of activity deprivation: the earlier the activity blockade is removed, the more neurons up-regulate PARV. This indicates that neuronal activity is the master regulator for PARV expression, suggesting that any experimental condition which alters the level of activity would alter the developmental expression of PARV. For instance, PARV increases in visual cortex with eye opening which results in higher activity and coherent activation patterns. Silencing one eye causes a reduction in PARV expression in the binocular portion of the contralateral visual cortex (Cellerino et al., 1992). The manipulation does not block cortical activity. It rather alters the balance of input from the two eyes indicating that patterns of activity might also be of importance. In fact, dark-rearing or binocular deprivation do not affect PARV expression, which indicates that sensory input arriving via thalamic fibers is not a major driving force (Cellerino et al., 1992).
Activity must be experienced before a given neuron commences PARV expression. Since action potential activity at this stage is still low (Klostermann and Wahle, 1999), we suggest that subthreshold events are sufficient for priming the subsequent PARV up-regulation. This is in line with earlier reports: PARV expression develops normally in cortex and hippocampus cultures explanted several days after birth (P8, Marty et al., 1996a; P5, Marty, 2000; P7-9, Vogt Weisenhorn et al., 1998). At this age many neurons likely have been primed to express PARV. By contrast, cultures deprived of activity from birth to 4 weeks completely failed to up-regulate PARV. Only a fraction (<0.1%) of PARV neurons remained detectable in long-term deprived OTC. PARV is thus more severely affected by the lack of activity than for instance NPY (Wirth et al., 1998), somatostatin (Marty et al., 1996b, 1997) and glutamic acid decarboxylase mRNAs (Patz et al., 2003) which remain in deprived OTC in much higher quantities. Moreover, the GAD expression instantly recovers from activity deprivation irrespective of the duration of deprivation.
The late onset deprivation revealed that PARV neuron numbers remain at the values present at the onset of blockade and do not continue to increase during the period of activity blockade. Activity is thus required throughout the developmental period for increasing PARV mRNA expression and neuron numbers to adult levels. This is peculiar finding, because it suggests that every individual PARV neuron must experience activity until the moment it starts to express the PARV mRNA. The alternative is that the nascent PARV expression remains sensitive to activity is unlikely because once the neurons have up-regulated PARV, the expression becomes constitutive and activity-independent. Also the GAD mRNA expression becomes constitutive with age in vitro (Patz et al., 2003). In contrast, the expression of NPY (Wirth et al., 1998) remains activity-dependent and declines with late-onset deprivation suggesting that each of the functional interneuron markers is controlled by specific sets of environmental factors.
Neurotrophins Accelerate PARV Expression
The rapid PARV increase during the third postnatal week in vivo is temporally correlated with the visually evoked peak in BDNF (Schoups et al., 1995). A BDNF overexpressing mouse displays a precocious structural maturation of PARV neurons and synapses and interneuronal function (Huang et al., 1999). In contrast, BDNF knockout mice reveal at P15 less cortical PARV-ir neurons than wildtype (Jones et al., 1994; Altar et al., 1997). However, at P28 they display normal numbers suggesting that in the absence of BDNF PARV expression is delayed, but not prevented from occuring, due to the general delay of differentiation in these mice (Marty et al., 1997). In contrast, striatal PARV expression remains reduced in BDNF knockout mice since it depends on BDNF delivered by cortical afferents (Altar et al., 1997). These results point to a pivotal role of neurotrophins for PARV expression.
We now show that exogenous and endogenous TrkB ligands accelerate PARV expression via Trk and MEK2 signalling. BDNF requires PI3 kinase signalling (Heumann, 1994), whereas NT-4 bypasses the PI3 kinase. The neurotrophins must be present already before the onset of PARV expression. In fact, the BDNF overexpression mouse has already higher than normal BDNF levels shortly after birth (Huang et al., 1999). BDNF also increases PARV neuron numbers in dissociated embryonic striatal cells when supplemented directly after plating (Mizuno et al., 1994) and in immature retina explants (Rickman, 1999). With age, the effectiveness in particular of BDNF declined and factor supplementation at ages beyond 14 DIV failed to promote PARV expression. A similar time window has been identified for the GAD expression which is promoted by TrkB ligands only during the first 10 DIV (Patz et al., 2003). BDNF also fails to promote PARV expression in hippocampal slices explanted at P8 (Marty et al., 1996a; Marty, 2000). PARV expression and neuron numbers were unaffected in visual cortex in vivo receiving BDNF and NT-4 infusions during the critical period (P20–28) although the PARV neurons up-regulate NPY and grow hypertrophic (Engelhardt et al., 2001). We do not believe that truncated Trk receptors or desensitization of full-length receptors are involved, because both TrkB ligands instantly increase NPY mRNA in PARV neurons in aged cocultures (Wahle et al., 2000). Moreover, adult mice overexpressing activated p21Ras as an ‘intracellular neurotrophin’ selectively in neurons reveal PARV staining intensity and neuron numbers similar to wild type (Heumann et al., 2000; own unpublished results).
Together, this argues for a temporally limited period in development during which TrkB ligands promote PARV expression. The factors must be available before the onset of PARV expression and in this aspect resemble the role of neuronal activity. However, neurotrophins act downstream of and could not substitute for neuronal activity. In particular NT-4 failed to activate PARV expression in deprived OTC, although it fully rescues NPY and GAD mRNA expression in the same condition (Wirth et al., 1998; Patz et al., 2003).
The Morphological Maturation of Basket and Chandelier Cells
Typical morphologies appear in OTC. Somata and axonal projection patterns were correctly positioned suggesting either that cortex-intrinsic factors are sufficient to drive class-specific differentiation, or that any decision concerning the morphological type has already been made before explantation. Further, all basket cells displayed the fast-spiking phenotype as early as the third week in OTC (Klostermann and Wahle, 1999) and at this stage less than half of the cells have commenced PARV expression. Thus, the morphological and physiological maturation of fast-spiking interneurons is not strictly coupled to PARV expression. Cell types, primary neurite pattern and typical presynaptic structures also appear in activity-deprived OTC suggesting that neuronal activity has no influence on the development of class-specific morphological features.
The exception was soma size. Larger PARV-ir somata were for instance found in the cortex of young rats receiving BDNF and NT-4 infusions (Engelhardt et al., 2001) and mice overexpressing activated p21Ras (own unpublished results) suggesting that cell volume depends on trophic factors. Interneurons grow larger in vitro (Obst and Wahle, 1995; Vicario-Abejon et al., 1995; Marty et al., 1996a,b), likely due to the unrestricted availability of endogeneous neurotrophins since for instance afferent systems competing for neurotrophins are missing. Indeed, PARV somata remained smaller in thalamocortical cocultures. Thalamic fibers terminate in middle cortical layers (Bolz et al., 1990) and thalamic cell growth depends exclusively on cortical NT-4 (Wahle et al., 2003). Middle layers also have the highest density of PARV neurons which suggests that PARV neurons compete with afferents for NT-4.
Soma size was smallest in activity-deprived OTC although it did not fall below in vivo size variation. Likely, the growth is mediated by NT-4 which is activity-independently expressed (Gorba et al., 1999; Ichisaka et al., 2003). By contrast, BDNF expression is drastically reduced in activity-deprived OTC. Possibly, the hypertrophy seen in active OTC was mediated by the additional availability of BDNF. Moreover, it appears as if volume regulation must proceed during the early period of differentiation because activity-deprived PARV somata fail to grow hypertrophic after recovery from deprivation, despite the fact that BDNF expression and release become instantly up-regulated with the onset of activity.
The work was supported by Deutsche Forschungsgemeinschaft SFB 509 ‘Neurovision’. We thank C. Riedel and Martina Diße for technical help.
Figure 1. Developmental expression of PARV mRNA. PCR revealed the steep increase in PARV mRNA in visual cortex in vivo (A, black triangles), the dramatic delay in spontaneously active OTC (A, grey squares), and the low expression in activity-deprived OTC (A, white circles). All values represent the mean with SEM normalized to the expression level at P2, DIV 2+ and DIV 2–, respectively, which were set to 1. (B) Representative PCR bands (with cycle numbers indicated above the lanes) comparing directly the PARV mRNA expression at DIV 20+ and P20, and DIV 50+ and P50. (C–E) Development of PARV mRNA expressing neurons in spontaneously active OTC at 20, 30 and 70 DIV revealed the inside-out progression of appearance; layer V is indicated. Scale bar = 100 µm.
Figure 2. Delayed PARV expression in OTC. At 20 DIV, PARV expression in OTC was far below the age-matched in vivo level which was set to 1. Cortical afferents (DIV 20+ VC-VC) failed to promote PARV (Mann–Witney U-test, P = 0.3 versus DIV 20+). Thalamic afferents (DIV 20+ VC-Th) increased cortical PARV mRNA (Mann–Witney U-test, P < 0.001 versus DIV 20+ and DIV 20+ VC-VC), but failed to elicit the age-matched in vivo level (Mann–Witney U-test, P < 0.001 versus P20). ***P < 0.001. For all bars: 8–12 PCRs, two independent cDNA libraries; the inset shows representative PCR bands for the four conditions.
Figure 3. The role of neuronal activity. PARV mRNA expressing neurons in 70 DIV active OTC (A) and 70 DIV activity-deprived OTC (B). Late-onset deprivation failed to down-regulate PARV mRNA expressing neurons (DIV 50+/14–; C) and did not alter the mRNA level (D; eight PCRs, two independent cDNA libraries). Scale bar = 100 µm (A–C).
Figure 4. Recovery from activity deprivation. OTC were activity-deprived for 0–8 (A), 0–14 (B) and 0–20 DIV (C) followed by return to normal medium and recovery of action potential activity for 20 DIV. When extending the initial period of deprivation, fewer and fewer neurons which managed to up-regulate PARV. PCR confirmed the inverse relation (D; for each bar 12 PCRs, three cDNA libraries); for instance, a 20 DIV period of deprivation followed by 20 DIV of recovery revealed PARV mRNA at levels similar to 30 DIV chronically deprived OTC. *P < 0.05, ***P < 0.001; Mann–Witney U-tests versus the DIV 30+ control condition which was set to 1. Scale bar = 100 µm (A–C). The inset in (D) shows representative PCR bands for the five conditions.
Figure 5. The role of neurotrophins. BDNF and NT-4 strongly promoted PARV mRNA expression when supplemented from 0 to 10 and 0 to 20 DIV to spontaneously active OTC. NT-3 had no significant effects (A; for each bar, eight PCRs, two cDNA libraries). At 20 DIV, the effectiveness of both TrkB ligands was reduced (A; for each bar, eight PCRs, two cDNA libraries). Both TrkB ligands failed to promote PARV expression when applied at ages beyond 14 DIV (B; for each bar, six PCRs, two cDNA libraries). The neurotrophin effects depended on Trk receptor activation (C; inhibitor K252a; 12 PCRs, three cDNA libraries), PI3 kinase signalling (C; inhibitor LY294002; 12 PCRs, three cDNA libraries) and MEK2 signalling (inhibitor U0126; 12 PCRs, two cDNA libraries), but not on MEK1 signalling (C; inhibitor PD98059; 12 PCRs, two cDNA libraries). As expected, exogeneous BDNF and NT-4 were unable to act in the precence of K252a and BDNF, but not NT-4 signalling depended on PI3 kinase (C; for each bar 12 PCRs, two cDNA libraries). All neurotrophins failed to rescue PARV mRNA expressing neurons in activity-deprived OTC at 30 DIV (D, 1) and PCR revealed mRNA levels not different from 30 DIV activity-deprived untreated OTC (for each bar, 12–16 PCRs, three or four cDNA libraries). *P < 0.05, ***P < 0.001, Mann–Witney U-tests versus the respective control conditions which in all graphs were set to 1. Scale bar = 100 µm (E).
Figure 6. PARV-immunoreactive structures in spontaneously active OTC at 50 DIV (A, upper layers; B, lower layers), in activity-deprived OTC at 40 DIV (C) and in a recovery culture (D; activity-deprivation from 0 to 5 DIV followed by recovery of activity for 20 DIV). Note the dramatic reduction in immunoreactivity in deprived OTC. However, few neurons always managed to express PARV in the absence of activity and a faint neuropil staining with perisomatic boutons was evident in their vicinity (E). In some neurons the axonal ramifications can be followed; the neurons shown are basket cells of layer V (F, G) in activity-deprived OTC. Arrows point to axons, asterisks in (B) mark basket terminals in layer V. Scale bars = 100 µm (A–D), 20 µm (E) and 50 µm (F, G).
Figure 7. PARV-immunoreactive neurons in OTC represented chandelier cells in supragranular layers (A, B), basket neurons with horizontal axons in layer III (C), neurons with arcade-type axons in layers III–V (D–F) and basket neurons with horizontal axons in layer V (G, H). Neurons (A, E) were reconstructed from 40 DIV spontaneously active OTC. Neurons (B–D, F–H) were reconstructed from 30–40 DIV activity-deprived OTC. Axons are indicated by arrows. Scale bar = 200 µm.
Figure 8. Morphological assessment of PARV-immunoreactive neurons. The number of primary dendrites (A) was the same at P40, DIV 40+ and DIV 40–. Soma size was influenced by neuronal activity (B). PARV neurons in DIV 15+ OTC were significantly larger than adult neurons in vivo (DIV 15+ versus P 70: Mann–Witney U-test, P < 0.001), grew significantly until DIV 40 (DIV 15+ versus DIV 40+: Mann–Witney U-test, P < 0.001) and remained at that size variation at DIV 70+. PARV somata in visual cortex-thalamus (V-T) cocultures were significantly smaller compared to monocultures (V-T DIV 70+ versus DIV 70+: Mann–Witney U-test, P < 0.001). Somata in activity-deprived OTC remained significantly smaller (e.g. DIV 40– versus DIV 40+: Mann–Witney U-test, P < 0.001). Late-onset deprivation largely failed to reduce soma size (DIV 20+/20– versus DIV 40–: Mann–Witney U-test, P < 0.001). By contrast, early deprivation followed by recovery yielded smaller somata (DIV 20–/20+ versus DIV 40+: Mann–Witney U-test, P < 0.0001) which despite recovery displayed the same size variation as chronically deprived neurons (DIV 20–/20+ versus DIV 40–: Mann–Witney U-test, P > 0.05).
Percentages of PARV neurons in spontaneously active and deprived OTC
| Age and experimental condition | Neurons (%) | n |
| Normal development | ||
| 15 DIV+ | 2.2 ± 1.4 | 15 |
| 20 DIV+ | 3.7 ± 1.6 | 15 |
| 30 DIV+ | 4.5 ± 1.1 | 23 |
| 50 DIV+ | 5.6 ± 1.4 | 18 |
| 70 DIV+ | 6.2 ± 1.4 | 18 |
| Chronic deprivation | ||
| 15 DIV– | 0.02 ± 0.005 | 9 |
| 20 DIV– | 0.02 ± 0.01 | 9 |
| 30 DIV– | 0.03 ± 0.02 | 11 |
| 50 DIV– | 0.04 ± 0.03 | 11 |
| 70 DIV– | 0.06 ± 0.02 | 8 |
| Age and experimental condition | Neurons (%) | n |
| Normal development | ||
| 15 DIV+ | 2.2 ± 1.4 | 15 |
| 20 DIV+ | 3.7 ± 1.6 | 15 |
| 30 DIV+ | 4.5 ± 1.1 | 23 |
| 50 DIV+ | 5.6 ± 1.4 | 18 |
| 70 DIV+ | 6.2 ± 1.4 | 18 |
| Chronic deprivation | ||
| 15 DIV– | 0.02 ± 0.005 | 9 |
| 20 DIV– | 0.02 ± 0.01 | 9 |
| 30 DIV– | 0.03 ± 0.02 | 11 |
| 50 DIV– | 0.04 ± 0.03 | 11 |
| 70 DIV– | 0.06 ± 0.02 | 8 |
Percentages of PARV neurons in spontaneously active and deprived OTC
| Age and experimental condition | Neurons (%) | n |
| Normal development | ||
| 15 DIV+ | 2.2 ± 1.4 | 15 |
| 20 DIV+ | 3.7 ± 1.6 | 15 |
| 30 DIV+ | 4.5 ± 1.1 | 23 |
| 50 DIV+ | 5.6 ± 1.4 | 18 |
| 70 DIV+ | 6.2 ± 1.4 | 18 |
| Chronic deprivation | ||
| 15 DIV– | 0.02 ± 0.005 | 9 |
| 20 DIV– | 0.02 ± 0.01 | 9 |
| 30 DIV– | 0.03 ± 0.02 | 11 |
| 50 DIV– | 0.04 ± 0.03 | 11 |
| 70 DIV– | 0.06 ± 0.02 | 8 |
| Age and experimental condition | Neurons (%) | n |
| Normal development | ||
| 15 DIV+ | 2.2 ± 1.4 | 15 |
| 20 DIV+ | 3.7 ± 1.6 | 15 |
| 30 DIV+ | 4.5 ± 1.1 | 23 |
| 50 DIV+ | 5.6 ± 1.4 | 18 |
| 70 DIV+ | 6.2 ± 1.4 | 18 |
| Chronic deprivation | ||
| 15 DIV– | 0.02 ± 0.005 | 9 |
| 20 DIV– | 0.02 ± 0.01 | 9 |
| 30 DIV– | 0.03 ± 0.02 | 11 |
| 50 DIV– | 0.04 ± 0.03 | 11 |
| 70 DIV– | 0.06 ± 0.02 | 8 |
Percentages of PARV neurons: late-onset deprivation and recovery
| Age and experimental condition | Neurons (%) | n |
| Late-onset deprivation | ||
| 5 DIV+/20 DIV– | 1.2 ± 0.3 | 15 |
| 20 DIV+/20 DIV– | 3.9 ± 1.5 | 11 |
| 30 DIV+/20 DIV– | 4.4 ± 0.8 | 10 |
| Recovery | ||
| 8 DIV–/20 DIV+ | 2.5 ± 1.1 | 10 |
| 14 DIV–/20 DIV+ | 0.6 ± 0.2 | 8 |
| 20 DIV–/20 DIV+ | 0.2 ± 0.05 | 19 |
| 30DIV–/20 DIV+ | 0.03 ± 0.005 | 9 |
| Age and experimental condition | Neurons (%) | n |
| Late-onset deprivation | ||
| 5 DIV+/20 DIV– | 1.2 ± 0.3 | 15 |
| 20 DIV+/20 DIV– | 3.9 ± 1.5 | 11 |
| 30 DIV+/20 DIV– | 4.4 ± 0.8 | 10 |
| Recovery | ||
| 8 DIV–/20 DIV+ | 2.5 ± 1.1 | 10 |
| 14 DIV–/20 DIV+ | 0.6 ± 0.2 | 8 |
| 20 DIV–/20 DIV+ | 0.2 ± 0.05 | 19 |
| 30DIV–/20 DIV+ | 0.03 ± 0.005 | 9 |
Percentages of PARV neurons: late-onset deprivation and recovery
| Age and experimental condition | Neurons (%) | n |
| Late-onset deprivation | ||
| 5 DIV+/20 DIV– | 1.2 ± 0.3 | 15 |
| 20 DIV+/20 DIV– | 3.9 ± 1.5 | 11 |
| 30 DIV+/20 DIV– | 4.4 ± 0.8 | 10 |
| Recovery | ||
| 8 DIV–/20 DIV+ | 2.5 ± 1.1 | 10 |
| 14 DIV–/20 DIV+ | 0.6 ± 0.2 | 8 |
| 20 DIV–/20 DIV+ | 0.2 ± 0.05 | 19 |
| 30DIV–/20 DIV+ | 0.03 ± 0.005 | 9 |
| Age and experimental condition | Neurons (%) | n |
| Late-onset deprivation | ||
| 5 DIV+/20 DIV– | 1.2 ± 0.3 | 15 |
| 20 DIV+/20 DIV– | 3.9 ± 1.5 | 11 |
| 30 DIV+/20 DIV– | 4.4 ± 0.8 | 10 |
| Recovery | ||
| 8 DIV–/20 DIV+ | 2.5 ± 1.1 | 10 |
| 14 DIV–/20 DIV+ | 0.6 ± 0.2 | 8 |
| 20 DIV–/20 DIV+ | 0.2 ± 0.05 | 19 |
| 30DIV–/20 DIV+ | 0.03 ± 0.005 | 9 |
References
Alcantara S, Ferrer I, Soriano E (
Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E (
Alcantara S, Soriano E, Ferrer I (
Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (
Baimbridge KG, Celio MR, Rogers JH (
Berchtold MW, Means AR (
Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H (
Bolz J, Novak N, Gotz M, Bonhoeffer T (
Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A (
Carder RK, Leclerc SS, Hendry SHC (
Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J (
Cellerino A, Siciliano R, Domenici L, Maffei L (
Cellerino A, Maffei L, Domenici L (
DeLecea L, del Rio JA, Soriano E (
Dreessen J, Lutum C, Schafer BW, Heizmann CW, Knopfel T (
Engelhardt M, DiCristo G, Berardi N, Maffei L, Wahle P (
Fairen A, DeFelipe J, Regidor J (
Gonchar Y, Burkhalter A (
Gorba T, Wahle P (
Gorba T, Klostermann O, Wahle P (
Heizmann CW (
Heumann R, Goemans C, Bartsch D, Lingenhohl K, Waldmeier PC, Hengerer B, Allegrini PR, Schellander K, Wagner EF, Arendt T, Kamdem RH, Obst-Pernberg K, Narz F, Wahle P, Berns H (
Ho YS, Howard AJ, Crapo JD (
Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S (
Ichisaka S, Katoh-Semba R, Hata Y, Ohshima M, Kameyama K, Tsumoto T (
Inokuchi K, Kato A, Hiraia K, Hishinuma F, Inoue M, Ozawa F (
Jones KR, Farinas I, Backus C, Reichardt LF (
Kawaguchi Y, Kubota Y (
Kawaguchi Y, Kubota Y (
Klapstein GJ, Colmers WF (
Klostermann O, Wahle P (
Marty S (
Marty S, Carroll P, Cellerino A, Castren E, Staiger V, Thoenen H, Lindholm D (
Marty S, Berninger B, Carroll P, Thoenen H (
Marty S, Berzaghi M da P, Berninger B (
Mione MC, Danevic C, Boardman P, Harris B, Parnavelas JG (
Mizuno K, Caranaha J, Nawa H (
Obst K, Wahle P (
Papadopoulos GC, Parnavelas JG, Buijs RM (
Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P (
Paul Blum B, Mann LL (
Porter LL, Rizzo E, Hornung JP (
Rickman DW (
Ross NR, Porter LL (
Schoups AA, Elliott RC, Friedman WJ, Black IB (
Sik A, Hajos N, Gulacsi A, Mody I, Freund TF (
Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio MR, Missiaen L, Robberecht W, Berchtold MW (
Vicario-Abejon C, Johe KK, Hazel TG, Collazo D, McKay RD (
Vogt Weisenhorn DM, Celio MR, Rickmann M (
Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B (
Wahle P (
Wahle P, Gorba T, Wirth MJ, Obst-Pernberg K (
Wahle P, DiCristo G, Schwerdtfeger G, Engelhardt M, Berardi N, Maffei L (
Wirth MJ, Obst K, Wahle P (