Abstract
Childhood trauma is associated with higher rates of both mood and anxiety disorders in adulthood. The exposure of rats to stressors during juvenility has comparable effects, and was suggested as a model of induced predisposition for these disorders. The neural cell adhesion molecule (NCAM) and its polysialylated form PSA-NCAM are critically involved in neural development, activity-dependent synaptic plasticity, and learning processes. We examined the effects of exposure to stressors during juvenility on coping with stressors in adulthood and on NCAM and PSA-NCAM expression within the rat limbic system both soon after the exposure and in adulthood. Exposure to stressors during juvenility reduced novel-setting exploration and impaired two-way shuttle avoidance learning in adulthood. Among naive rats, a development-related decrease of about 50% was evident in the PSA-NCAM to NCAM expression ratio in the basolateral amygdala, in the CA1 and dentate gyrus regions of the hippocampus, and in the entorhinal cortex. In juvenile-stressed rats, we found no such decrease, but rather an increase in the polysialylation of NCAM (∼50%), evident soon after the exposure to juvenile stress and also in adulthood. Our results suggest that exposure to stressors during juvenility alters the maturation of the limbic system, and potentially underlies the predisposition to exhibit stress-related symptoms in adulthood.
Similar content being viewed by others
INTRODUCTION
Accumulating evidence indicates that chronic stress or continuous glucocorticoid elevation in adult rats affects the functioning of neural cell adhesion molecules (NCAMs), particularly NCAM and its polysialylated form PSA-NCAM (Sandi, 2004). NCAMs are membrane-bound glycoproteins of the immunoglobulin superfamily of adhesion molecules, which mediate cell–cell interactions; by interacting with cytoskeletal components, they can activate specific intracellular signaling pathways (Cremer et al, 1997). These molecules play a pivotal role in neural development and regeneration, and are strongly implicated in synaptic plasticity and memory formation processes (Schachner, 1997; Kiss et al, 2001; Sandi, 2004). NCAMs can be polysialylated on their extracellular domains by attachment of long α2, 8-linked polysialic acid (PSA) homopolymer chains (Hoffman et al, 1982; Friedlander et al, 1985). This posttranslational modification, which is crucial to their morphoregulatory functions (Dey et al, 1999), weakens the adhesive properties of NCAM (Schachner, 1997) and is believed to inhibit NCAM-mediated interactions (Doherty and Walsh, 1991; Cremer et al, 1997; Schachner, 1997; Sandi et al, 2001). Thus, it was suggested that PSA-NCAM acts as a plasticity promoter by decreasing overall cell adhesion, thereby allowing structural remodeling to occur (Rutishauser and Landmesser, 1996), whereas NCAM acts as a stability promoter (Rønn et al, 2000). Normal brain development also involves temporal and spatial alterations in the expression of these NCAMs (Edelman, 1984; Rutishauser and Jessell, 1988; Rutishauser, 1989).
During early development, PSA-NCAM is widely expressed, but in the adult brain its expression is confined to a few restricted areas characterized by a high level of structural remodeling, such as the hippocampal formation (Rønn et al, 2000). The transient re-expression of PSA-NCAM was also associated with activity-dependent synaptic remodeling (Rønn et al, 1998, 2000). Throughout development, expression of the plasticity-promoting PSA-NCAM decreases, whereas the relative expression of the nonpolysialylated, stability-promoting NCAM increases (Rønn et al, 1998).
Developmentally regulated changes in PSA-NCAM expression influence fundamental morphogenetic processes, such as neuronal migration, neurite elongation, and synaptogenesis (Doherty and Walsh, 1991; Rutishauser and Landmesser, 1996) by modulating neuron–neuron (Nybroe and Bock, 1990), neuron–astrocyte (Keilhauer et al, 1985), and neuron–substratum (Covault, 1989) recognition and adhesion (Rutishauser, 1990). NCAM expression and its posttranslational polysialylation, which decreases the adhesive strength of NCAM, are critically involved in memory formation and activity-dependent synaptic remodeling processes (Doyle et al, 1992b; Lüthi et al, 1994; Rose, 1995; Arami et al, 1996; Becker et al, 1996; Foley et al, 2000; Welzl and Stork, 2003). Learning is also characterized by altered expression and glycosylation of NCAM (Doyle et al, 1992a; Rusakov et al, 1994; O'Connell et al, 1997; Tiunova et al, 1998; Sandi et al, 2004). Targeted disruption of the NCAM gene resulted in learning deficits (Cremer et al, 1994; Stork et al, 2000) accompanied by alterations in emotional/motivational behavior and by hyperresponsiveness of the serotonergic system (Stork et al, 1999, 2000).
A series of studies showed that chronic stress protocols known to produce cognitive and neural alterations (McEwen, 1999) markedly affected the expression of NCAMs in the hippocampus and other brain areas (Sandi, 2004). Chronic restraint stress (21 days × 6 h) diminished NCAM mRNA and protein expression in the hippocampus and other brain regions (Sandi et al, 2001; Venero et al, 2002); it did not alter the expression of NCAM-180 kDa isoform mRNA (Venero et al, 2002) but did specifically reduce the expression of NCAM-140 kDa isoform protein (Touyarot and Sandi, 2002). This reduction in NCAM-140 kDa expression levels was also observed following a social stress procedure, and seemed to be particularly pronounced in stressed rats exhibiting impaired water maze spatial learning as well as increased levels of corticosterone (CORT) (Touyarot et al, 2004).
Polysialylation of NCAM is also affected by stress. A chronic 3-week restraint stress protocol, which decreased cell proliferation in the hippocampus dentate gyrus (DG) (Sandi, 2004), also increased PSA-NCAM expression in the DG (Sandi et al, 2001) and in the CA3 section of the hippocampus (Nacher et al, 2004; Sandi, 2004), although downregulating PSA-NCAM expression in the amygdala complex (Cordero et al, 2005).
Though much is known about the effects of chronic stress on the functioning of NCAM in adult rats, relatively little is known about how these molecules are affected by early life stress (ELS) (Sandi, 2004). Recent evidence indicates that both prenatal and perinatal stress exposure causes profound downregulation of hippocampal and cortical NCAM expression, as well as long-lasting cognitive alterations in the adult offspring, with a parallel reduction in brain-derived neurotrophic factor (BDNF) and in the synaptic marker synaptophysin (Koo et al, 2003).
Stressful and traumatic experiences early in life predispose individuals to develop mood and anxiety disorders, and were shown to be associated with multiple endocrine and anatomical changes in the neuronal circuits that are critically involved in modulating stress responses, emotional processing, and learning within the limbic system (Heim and Nemeroff, 2001; Heim et al, 2004; Nemeroff, 2004).
Most ELS rodent models focus on the perinatal to preweaning periods and involve some form of maternal deprivation or separation (for a review, see Sanchez et al, 2001). However, we focused on an alternative ELS-sensitive period in the rat ontogeny, namely the juvenile stage (∼28 days), the earlier phase of the adolescent/postweaning to prepubertal period (Avital and Richter-Levin, 2005; Tsoory and Richter-Levin, 2006; Avital et al, 2006; Tsoory et al, 2007). During the adolescent period (21–42 days), substantial maturational processes occur in the rat limbic system, including in the hippocampus and amygdala-based neurocircuits (for a review, see Spear, 2000).
During the juvenile period, the hypothalamic-pituitary-adrenal (HPA) axis response reaches its developmental asymptote (Vazquez, 1998); however this response lasts considerably longer than in adults (Vazquez, 1998; Romeo et al, 2004). Romeo et al (2004) suggested that this slower shutoff of the HPA axis during juvenility might derive from there being less centrally mediated feedback from various underdeveloped forebrain limbic regions at this age.
Exposure to stressors during juvenility was reported to produce more pronounced effects than exposure at earlier or later ages, affecting object exploration in adulthood (Einon and Morgan, 1977), fluid intake (McGivern et al, 1996), and adulthood social and nonsocial behaviors associated with disregulation of endogenous opioid system development (Van den Berg et al, 1999a, 1999b, 1999c, 2000).
Several studies suggested that exposure to stressors during juvenility affects limbic system development, which may lead to enduring effects on coping with stressors in adulthood. Adult rats chronically exposed to variable stressors throughout juvenility had an enhanced acoustic startle response similar to patients with posttraumatic stress disorder (PTSD) (Maslova et al, 2002). Exposure to acute stressors during juvenility produces increased vulnerability to stressful events in adulthood, resulting in an augmented response to adverse experiences (Avital and Richter-Levin, 2005). In addition, a short-term juvenile exposure to variable stressors produced two types of impaired avoidance learning reminiscent of symptoms of both mood and anxiety disorders (Tsoory and Richter-Levin, 2006; Tsoory et al, 2007).
Exposure to the juvenile short-term variable stressor protocol transiently delayed body weight gain (Horovitz et al, 2007). In comparison with control (unexposed) rats, juvenile-stressed rats exhibited less body weight gain when examined soon after the exposure (at 34 days of age). However, later on during the maturation process (at 41, 48, 55, and 62 days of age), this difference was not evident. A two-way analysis of variance (ANOVA) of percent body weight gain for age and groups (control, juvenile-stressed) revealed a main effect for age (F(3,14)=1642.61; p<0.01), but not for groups (F(1,14)=2.94; NS) and not for the interaction between age and groups (F(3,14)=1.09; NS). Subsequent t-tests compared percent body weight gain between the groups at each of the noted ages and found a significant difference only at the age of 34 days (C=170.81±2.23; J=162.73±0.93; t(14)=3.34; p<0.01), indicting that though the stressor affected body weight gain in the short run, in the long run juvenile-stressed rats continue to develop normally in terms of their body weight gain (Horovitz et al, 2007). This transient effect may be owing to the timing of the exposure to stressors, ie juvenility, close to their growth spurt at 4–5 weeks (Spear, 2000), which may have overshadowed any stress-related body weight reduction. Alternatively, the lack of a long-term effect on body weight may be due to the fact that stress-related reductions in body weight are prevalent following chronic stress procedures (eg Bekris et al, 2005; Cordero et al, 2005), but not following acute stress procedures (eg Mizoguchi et al, 2001; Retana-Marquez et al, 2003). Mizoguchi et al (2001) suggested that this difference might be associated with the differential effects these procedures have on HPA axis responses.
Furthermore, exposure to stressors during juvenility affected the HPA axis response to stressors in adulthood (Tsoory et al, 2004). A comparison of the baseline CORT levels (μg/ml) of adult rats (60 days old) was undertaken between naive (N) rats and rats exposed to a brief predator scent stressor during juvenility (J, at 28 days of age) or during adulthood (A, at 59 days of age), or during both juvenility and adulthood (J+A, at 28 days of age and again at 59 days of age) (for a full description of the stress procedure and groups see Tsoory et al, 2007). An ANOVA indicated a significant main effect for these groups (F(3,30)=18.36; p<0.05). Scheffe post hoc tests revealed that compared to the baseline CORT levels of naive rats (N=139.97±28.18; n=10), only rats which were exposed to the stressor in adulthood alone exhibited significantly (p<0.05) increased baseline CORT levels (A=263.11±42.28; n=8), whereas rats which were exposed to the stressor only during juvenility (J=16.76±2.42; n=8) and those exposed to it during both juvenility and adulthood (J+A=25.70±4.59; n=8) exhibited significantly lower baseline CORT levels (p<0.05). The baseline CORT levels of J and J+A rats did not differ from each other. Taken together, with the substantial difference found between the baseline CORT levels of A and J+A rats, it was suggested that the juvenile exposure altered the consequences of the later, adulthood, exposure. Similar differences in HPA axis responses were recently reported among PTSD patients (Santa-Ana et al, 2006). Following a physical challenge (the cold pressor task), adult PTSD patients who experienced childhood trauma exhibited a blunted HPA axis response (ie significantly lower cortisol levels at baseline and throughout the 2 h following the challenge) compared to both control subjects and PTSD patients who were traumatized in adulthood. Control subjects and PTSD patients traumatized in adulthood did not differ in their HPA axis responses. The authors suggested that this might indicate that childhood trauma confers a greater impairment on HPA axis responses than does traumatization later in life (Santa-Ana et al, 2006).
Utilizing our ‘juvenile stress’ animal model (Tsoory and Richter-Levin, 2006), the present study sought to examine whether the ‘juvenile stress’ protocol that induces a predisposition for impaired coping with stressors in adulthood also affects NCAM and PSA-NCAM development-related alterations. To this end, we examined the effects of exposure to ‘juvenile stress’ on NCAM and PSA-NCAM expression both soon after the exposure and in adulthood, whereas reconfirming our previous findings regarding altered emotional responses in adult ‘juvenile-stressed’ rats.
MATERIALS AND METHODS
Study Design
To evaluate the consequences of exposure to stressors during juvenility, on coping with stressors in adulthood, and to assess development-related changes in NCAM and PSA-NCAM expression, rats were randomly exposed to a short-term stress protocol during juvenility (juvenile-stressed) (27–29 days of age) or not exposed (naive). In each of these conditions, about a third of the rats were designated for behavioral assessment of coping with stressors in adulthood, to confirm our previous long-term behavioral effects findings. From the remaining two thirds, limbic brain tissues were collected for later immunoblotting. Collection took place either soon after the exposure, at the age of 33 days, or later in adulthood at the age of 9 weeks. Thus, limbic brain regions were harvested from the following four groups:
-
1)
Juvenile-stressed rats (J-S): 33-day-old rats exposed to a short-term stress protocol during juvenility (27–29 days of age); n=8.
-
2)
Juvenile naive rats (J-N): 33-day-old naive rats, not exposed to adverse conditions; n=8.
-
3)
Adult juvenile-stressed rats (A-JS): 9-week-old rats previously exposed to the stress protocol during juvenility (27–29 days of age); n=10.
-
4)
Adult naive rats (A-N): 9-week-old naive rats, not exposed to adverse conditions; n=10.
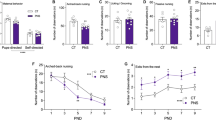
As depicted in Figure 1, to ascertain the long-term behavioral effects of exposure to stressors during juvenility, we verified its effect on novel-setting exploration in all the adult rats (A-N: n=20; A-JS: n=24). Following this test, about a half of all the adult rats (A-N: n=10; A-JS: n=14) were trained in the two-way shuttle avoidance task to confirm previous results regarding impaired learning under stressful conditions. No tissue was collected from these rats. Twenty-four hours following the exploratory assessment, limbic brain regions were collected from the adult rats, which were not trained in the avoidance task: the above-noted groups 3 (A-JS: n=10) and 4 (A-N: n=10).
Study design: Following weaning at the age of 21 days, 22-day-old male Sprague–Dawley rats were supplied by Harlan Laboratories (Jerusalem) and, after 5 days of acclimation to the vivarium, were randomly assigned to be exposed to stressors during juvenility (juvenile-stressed: black textboxes and arrows) or not exposed (naive: white textboxes and arrows). When the rats were 33-days old, limbic brain regions were harvested from some members of each group (J-S, n=8; J-N, n=8). To ascertain the long-term behavioral effects of exposure to stressors during juvenility, we verified its effect on novel-setting exploration by the remaining rats when they reached adulthood (9 weeks) (A-N, n=20; A-JS, n=24). Immediately following this test, about half of the adult rats (A-N, n=10; A-JS, n=14) were trained in the two-way shuttle avoidance task to ascertain impaired learning under stressful conditions. No tissue was collected from these rats, whereas from the other half, which were not trained in the shuttle avoidance task (A-JS, n=10; A-N, n=10), limbic brain regions were collected 24 hours after the exploration assessment.
Animals
Sixty male Sprague–Dawley rats, 22 days old, weighing 35–49 g on delivery from Harlan Laboratories Jerusalem, were maintained for the entire duration of the experiment in the laboratory's vivarium. Vivarium conditions: lighting, 12 h light–dark cycle, lights on at 0700; temperature, 22±2°C; cages, floor area of 2653.5 cm2 (610 × 435 mm), height, 215 mm (Model # 2000P, TECNIPLAST S.p.a., Varese, Italy), 3–4 rats per cage, bedding of sterilized pine sawdust; Diet: ad libitum water and solid food pellets (Teklad Global Diet 2018S, Harlan Teklad Ltd, WI, USA).
All stress procedures and behavioral assessments were conducted in designated experiment rooms away from the vivarium, were approved by the institutional animal care committee, and adhered to the NIH Guide for the Care and Use of Laboratory Animals.
‘Juvenile Stress’ Procedure
Research indicates that variable stressor protocols elicit stronger stress responses than acute or repeated stressor protocols in adult rats (Garcia-Vallejo et al, 1998; Prieto et al, 2003; Simpkiss and Devine, 2003), and that prepubertal male rats exhibit an enhanced stress response as compared with adults (Romeo et al, 2004). On the basis of these findings, we designed a juvenile short-term variable stressor protocol (Tsoory and Richter-Levin, 2006; Tsoory et al, 2007).
Rats in the juvenile stress group were exposed to a different stressor every day for 3 days (see below). Stress exposure took place during juvenility (ages 27–29 days) at approximately midday (1200–1400) in designated experimental rooms (a different room each day) away from the vivarium. Each of the stressors was chosen for its well-documented effects on adult rats.
-
Day 1 (aged 27 days) Forced swim: 10 min forced swim in an opaque circular water tank (diameter 0.5 m; height 0.5 m; water depth 0.4 m), water temperature 22±2°C (adapted from Avital et al, 2001). Similar forced swim protocols affected the stress-response mechanisms of adult rats (Hall et al, 2001).
-
Day 2 (aged 28 days) Elevated platform: three 30 min trials; intertrial interval(ITI), 60 min in the home cage. Elevated platform, 12 × 12 cm at a height of 70 cm above floor level, located in the middle of a small closet-like room (adapted from Maroun and Richter-Levin, 2003). Similar elevated platform protocols affected the stress-response mechanisms of adult rats (Degroot et al, 2004; Ebner et al, 2004).
-
Day 3 (aged 29 days) Foot-shock or restraint stressors: rats were randomly subjected to a short foot-shock session or to 2 hours of restraint. This was carried out to minimize possible attenuation of stress-induced increase in anxiety owing to similarities between the electric foot shocks used in the juvenile stress protocol and those used later in adulthood in the two-way shuttle avoidance task. No differences were observed between rats exposed to the foot-shock stressor or the restraint stressor procedures in any of the measured indices.
Foot Shock
A 3- min session of six unconditioned electric foot shocks (1 s, 0.8 mA); ITI: 29 s; apparatus: a small cube-like chamber (31 × 31 × 31 cm) with a metal grid floor connected to a computer-controlled electrical shocker device (Solid State Shocker/Scrambler, Model no. 113–33, Lehigh Valley Electronics Ltd, Lehigh Valley, PA, USA). Similar foot shock procedures affected the stress-response mechanisms of adult rats (Li and Sawchenko, 1998; Passerin et al, 2000; Pezzone et al, 1993, 1992; Rassnick et al, 1998).
Restraint
Rats were placed in a metal mesh-restraining box (11 × 5 × 4 cm) that prevented forward-backward movement and limited side-to-side mobility, but did not discomfort the animal in any other way. Rats remained in the restraining box for 2 h at 25°C under dim illumination. Similar restraint procedures affected the stress-response mechanisms of adult rats (Pace et al, 2005; Martijena et al, 2002).
Protocols were applied in parallel to rats in the stress group, so as not to isolate any rat in its home cage. Upon completion of the each of the first two stress procedures, rats were returned to their home cage and were not handled until the next day. Following completion of the day 3 stress procedure, rats were returned to their home cage and were not handled again until either brain regions harvesting at the age of 33 days or behavioral assessments in adulthood at the age of 9 weeks, except for weekly cage maintenance.
Behavioral Assessments in Adulthood
In adulthood, at the age of 9 weeks, the novel-setting exploratory behavior of rats and their learning under stressful conditions (ie during a two-way shuttle avoidance task) were assessed. This was carried out by a group of three experimenters who were aware of which group each rat belonged to, as they had to run the tests in semirandom order to avoid a ‘group order effect’. However, data collection was performed by an automated, PC-controlled, system.
-
Novel-setting exploration. Rats were placed in the two-way shuttle avoidance apparatus described below, although it was in an inoperative mode, and were allowed to explore both compartments for a total of 10 min. Crossingover between compartments provided an index of exploratory behavior. If a rat did not visit the adjacent compartment within 5 min, it was gently, manually directed through the door. The same was performed if the rat failed to cross back into the first compartment within another 5 min. An observing experimenter monitored the exploratory shuttles of the rats. Only voluntary shuttles were counted.
-
Two-way shuttle avoidance task. Immediately following the exploratory behavior assessment, about a half of all adult rats (as noted above in ‘Study Design’ and in Figure 1) were trained in the two-way shuttle avoidance task in a single 100-trial session. Apparatus: The two-way shuttle avoidance box, placed in a dimly lit, ventilated, sound-attenuated cupboard, is a rectangular chamber (60 × 26 × 28 cm) divided by an opaque partition with a small passage (10 × 8 cm) connecting two equally sized, side-by-side, cube-shaped compartments. The metal grid floors of both compartments are weight sensitive, with microswitches transmitting information on the rat's location to a computer-controlled and automated data collection program. This program controls both conditioned stimulus (CS) presentations (a tone produced by loudspeakers located on the distal walls of the compartments) and unconditioned stimulus (US) electric shock deliveries (electrical shocker device: Solid State Shocker/Distributor, Model # 113–33, Coulborn Instruments Inc., Lehigh Valley, PA, USA) as well as recording the rats' responses. Procedure: One session of 100 ‘trace conditioning’ trials. CS: 10 s tone presentation; US: immediately following termination of the CS, an electric shock (0.5 mA) was delivered for a maximum of 10 s; ITI: 60±12 s. Rats could produce one of three responses: (1) Avoidance: shuttling to the adjacent compartment upon hearing the CS-tone; thereafter, the tone stopped, an ITI commenced, and the rat avoided the electric shock. (2) Escape: shuttling to the adjacent compartment although the shock was on; the shock terminated and an ITI commenced. (3) Escape failure: failing to move to the adjacent compartment; the ITI commenced at the completion of the 10 s foot shock, so the rat was subjected to the full duration of the electric shock.
Limbic Brain Regions Harvesting
Rats were decapitated by a guillotine (Stoelting, Wood Dale, IL, USA) and the following brain regions were collected based on rat brain coordinates (Paxinos and Watson, 1998): basolateral amygdalae (BLAs), left and right separately (Figure 2a), the CA1 and DG subregions of the hippocampus, and the entorhinal cortex (EC).
(a) BLA dissection: The dashed lines represent the dissection procedure employed on a thick (∼1 mm) coronal slice cut ∼6 mm rostal of interaural plane (indicated by the anterior ‘end’ of the cerebellum). (1) Cutting the ventral part of the slice just below the base of the optic tract; (2) cutting orthogonally to the first cut and in parallel to the corpus callosum about 2 mm medially to the rhinal fissure; (3) cutting out a small isosceles (∼1.5 mm) right triangle. (b) Monitoring protein concentrations across groups and brain regions: α-extracellular signal-regulated kinase type II (α-ERKII) staining (1:1000, II: α-Rabbit 1:10 000, cell-signaling; 12% acrylamide) across groups and brain regions indicating equal amounts of loaded protein.
Immediately following decapitation, the whole brain was removed from the skull and placed on an ice-cooled glass dish. First, the rostal hippocampus was dissected out and then the dorsal CA1 and DG sections were separated. Then a thick (∼1 mm) coronal slice was cut ∼6 mm anterior of the interaural plane (indicated by the rostal end of the cerebellum), and the BLAs were cut out as indicated in Figure 2a. This involved: (1) cutting the ventral part of the slice just below the base of the optic tract; (2) cutting orthogonally to the first cut and in parallel to the corpus callosum about 2 mm medial to the rhinal fissure; (3) cutting out a small isosceles (∼1.5 mm) right triangle. The sample from the EC (a small, ∼1.5 mm, isosceles triangle) was then dissected from the posterior ventral part of the cerebral cortex.
The regions were immediately homogenized in an ice-cold glass/Teflon homogenizer (885502-0019; Kontes Glass Company, Vineland, NJ, USA) using 50 Teflon/glass mortar strokes in 300 μl of ice-cold lysis buffer (50 mM Tris-HCl, 1 mM EDTA, 1.5 mM MgCl2, 0.8 M NaCl, 10% glycerol, pH 7.2–7.4), to which the following protease inhibitors were freshly added: 1 mM phenylmethylsulphonylfluoride, 1 mM dithiothreitol, 1 mM sodium orthovanadate, 1 μg/ml leupeptine, 1 μg/ml aprotinin, and 50 mM NaF. A total of 30 μl of each lysate was saved for further protein concentration by Bradford analysis. 5 × protein Laemmli denaturation buffer (17.5 ml of 5 × concentrated stacking buffer, pH 6.8, 7.5 ml glycerol, 10% sodium dodecyl sulfate, 2.33 g bromophenol blue) was added to each remaining lysate, thoroughly mixed and denatured for 5 min at 95°C. The denatured proteins were stored at −80°C for further analysis.
Immunoblotting
Protein concentration was monitored using Bradford assay, and equal amounts of loaded protein were verified using α-extracellular signal-regulated kinase type II (ERKII) staining (1:1000, II: α-Rabbit 1:10 000, cell-signaling; 12% acrylamide). No differences were observed between the groups in terms of ERKII concentrations in any of the examined regions (Figure 2b).
Individual samples from each region of each rat (100–120 μg/μl of lysates) were loaded onto 8% sodium dodecyl sulfate-polyacrylamide gel gels. Following electrophoresis, the gels were transferred by wet transfer tanks to nitrocellulose membranes and stained against α-NCAM (NCAM (H-94) Santa Cruz, sc8305-1:500; α-Rabbit 1:7000, Chemicon) and α-PSA-NCAM (PSA-NCAM: Chemicon MAB5324-1:1000, α-mouse 1:10000, Chemicon). The membranes were ECL-stained by luminol and β-coumaric acid. For each lane, the mean band intensity was relatively assessed using the NIH Scion program for totalNCAM (based on all three major NCAM isoforms: 120, 140, and 180 kDa) and for totalPSA-NCAM (based on all three PSA-NCAM isoforms: 140, 180, and 220 kDa).
Statistical Analysis
The results are expressed as mean±SEM. Differences between groups in terms of exploratory behaviors were determined by Student's t-tests. Differences between the responses of each group in the two-way shuttle avoidance task were determined using a two-way ANOVA for groups and for blocks of 10 trials (repeated measure analysis) followed by Student's t-tests per block. The effects of exposure to stressors during juvenility and development on totalNCAM and totalPSA-NCAM expression, indexed by blotting mean band intensity, were determined within each of the brain regions using a two-way ANOVA for ‘juvenile stress’ and development followed by subsequent Student's t-tests with Bonferroni corrections.
RESULTS
The Effects of Exposure to Stressors during Juvenility on Adulthood Stress Responses
We have previously demonstrated that exposure to stressors during juvenility has long-lasting effects on rats' ability to cope with stressful situations in adulthood (Avital and Richter-Levin, 2005; Tsoory and Richter-Levin, 2006; Tsoory et al, 2007). The current study is aimed to assess the effects of exposure to stressors during juvenility on development-related changes in expression levels of NCAM and PSA-NCAM. Accordingly, we first verified the efficacy of the exposure to stressors during juvenility in the present study by examining its effects on adulthood coping responses by using stressful challenges, namely, novel-setting exploration and avoidance learning.
As in previous studies, exposure to stressors during juvenility significantly reduced adulthood exploratory behavior. Compared with adult naive rats (A-N, n=20), adult juvenile-stressed rats (A-JS, n=24) exhibited significantly less exploration of the novel setting, namely the shuttle box before avoidance learning (t(42)=5.29; p<0.01) (Figure 3a). Similarly, exposure to stressors during juvenility significantly impaired adulthood learning under stressful conditions, that is, it reduced the rates of avoidance responses and increased the rates of escape failures in the learning of the two-way shuttle avoidance task. Repeated measure analysis of avoidance shuttles per block of 10 trials revealed a significant main effect for groups (F(1,17)=14.20; p<0.01), for blocks (F(3,57)=15.89; p<0.01), and for their interaction (F(3,57)=3.65; p<0.05). Additional t-tests per block showed that adult juvenile-stressed rats (A-JS, n=14) performed significantly fewer avoidance shuttles than naive adult rats (A-N, n=10) in blocks 1, 3, and 5–10. Accordingly, the number of total avoidance shuttles by A-JS rats was significantly lower than that of naive adult rats (t(22)=3.86; p<0.01) (Figure 3b). No differences were found between these groups in terms of escape responses while learning the two-way shuttle avoidance task, but A-JS rats had higher rates of escape failure than naive adult rats. Repeated measure analysis for escape failures per block of 10 trials revealed a significant main effect for groups (F(1,17)=10.19; p<0.01) but not for blocks (F(3,51)=0.99; NS), nor for their interaction (F(3,51)=0.85; NS). Additional t-tests per block showed that A-JS rats (n=14) exhibited significantly higher rates of escape failure than A-N rats (n=10) in blocks 1–10. Accordingly, the number of total escape failure by A-JS rats was significantly higher than for A-N rats (t(22)=4.59; p<0.001) (Figure 3c).
The effects of exposure to stressors during juvenility on novel-setting exploration and two-way shuttle avoidance learning in adulthood: exposure to stressors during juvenility significantly impaired coping with stressors in adulthood. Compared with adult naive rats (A-N), adult juvenile-stressed rats (A-JS) exhibited significantly fewer exploratory shuttles in a novel setting before avoidance learning (a); performed significantly fewer avoidance shuttles (b); and exhibited an increased rate of escape failures (c) whereas learning the two-way shuttle avoidance task. **Significantly different from naive rats, p<0.01.
Alteration of TotalNCAM and TotalPSA-NCAM Development-Related Changes in Expression Following Exposure to Stressors during Juvenility
To evaluate the effects of exposure to stressors during juvenility on development-related changes in the expression of totalNCAM and totalPSA-NCAM, a two-way ANOVA was conducted on each of the monitored regions of the limbic system: the BLA (left and right pooled, although no laterality effect was evident), CA1, DG, and EC. The two-way ANOVAs compared juvenile-stressed rats (n=18 (J-S, n=8+A-JS, n=10)) with naive rats (n=18 (J-N, n=8+A-N, n=10)) to examine the effects of exposure to stressors during juvenility on totalNCAM and totalPSA-NCAM, and compared juvenile rats (n=16 (J-S, n=8+J-N, n=8)) with adult rats (n=20 (A-JS, n=10+A-N, n=10)) to examine the effects of development on these NCAMs.
Effects of Exposure to Stressors during Juvenility on Development-Related Alterations in the Expression Levels of TotalNCAM
Figure 4 depicts the effects of both exposure to stressors during juvenility and development on totalNCAM levels in each of the monitored regions of the limbic system. A two-way ANOVA for exposure to stressors during juvenility and for development indicated that in each of the monitored regions totalNCAM expression levels were significantly affected by juvenile stress exposure (BLA (F(1,32)=208.69; p<0.01); CA1 (F(1,32)=75.08; p<0.01); DG (F(1,32)=131.21; p<0.01); EC (F(1,32)=107.04; p<0.01)) and by development (BLA (F(1,32)=154.60; p<0.01); CA1 (F(1,32)=26.19; p<0.01); DG (F(1,32)=184.45; p<0.01); EC (F(1,32)=187.33; p<0.01)). The interaction between juvenile stress and development was also significant for totalNCAM in all the monitored limbic regions (BLA (F(1,32)=234.12; p<0.01); CA1 (F(1,32)=59.43; p<0.01); DG (F(1,32)=226.94; p<0.01); EC (F(1,32)=162.60; p<0.01)).
The effect of exposure to stressors during juvenility and the effect of development on totalNCAM levels across the limbic system. A two-way ANOVA indicated that in each of the monitored regions: the BLA, the CA1 and DG regions of the hippocampus, and the EC, totalNCAM levels were significantly affected by exposure to stressors during juvenility, by development, and by the interaction of exposure to stressors during juvenility with development. The insets depict representative bands of NCAM isoforms across groups (juvenile naive, J-N; juvenile-stressed, J-S; adult naive rats, A-N; and adult juvenile-stressed rats, AJ-S) and in the examined limbic regions: BLA, CA1, DG, and EC. At juvenility, totalNCAM levels did not differ significantly between J-S (n=8) and J-N (n=8) rats in the BLA, CA1, and EC, whereas, in the DG, there was a mild but significant increase in the totalNCAM levels of J-S rats compared to J-N rats. In adulthood, the totalNCAM levels of A-JS rats (n=10) were significantly and substantially lower than those of A-N rats (n=10) in all examined limbic regions. **significantly different from age-matched naive rats, p<0.01. *significantly different from age-matched naive rats, p<0.05. Among naive rats, totalNCAM levels increased significantly during maturation from juvenility to adulthood in all examined limbic regions. However, such development-related increases did not occur among juvenile-stressed rats in the CA1, DG, and EC regions, whereas, in the BLA, a significant decrease was observed. ##significantly different from juvenile rats of the same group, p<0.01.
Examining the effect of exposure to juvenile stress on totalNCAM expression levels in each age group
Subsequent t-tests with Bonferroni corrections were conducted to compare totalNCAM expression levels between juvenile stress-exposed and naive rats at juvenility and adulthood. These comparisons indicated that, during juvenility, totalNCAM levels did not differ significantly between juvenile-stressed rats (J-S; n=8) and naive rats (J-N, n=8) in the BLA (t(14)=0.82; NS), CA1 (t(14)=0.49; NS), and EC (t(14)=1.39; NS), whereas in the DG, there was a mild yet significant increase in the totalNCAM levels of J-S rats compared to J-N rats (t(14)=2.81; p<0.05)). However, in adulthood, the expression levels of totalNCAM in adult juvenile-stressed rats (A-JS, n=10) appeared to be significantly lower than those of naive adult rats (A-N, n=10) in all examined regions (BLA (t(18)=18.85; p<0.01); CA1 (t(18)=18.80; p<0.01); DG (t(18)=18.15; p<0.01); EC (t(18)=20.19; p<0.01)).
Examining the effect of development on the totalNCAM expression levels among juvenile-stressed vs naive rats
Additional t-tests with Bonferroni corrections compared juvenility and adulthood for rats exposed to stressors during juvenility and naive rats, and indicated that although among naive rats the totalNCAM levels significantly increased during maturation from juvenility into adulthood (BLA (t(16)=16.98; p<0.01); CA1 (t(16)=13.28; p<0.01); DG (t(16)=18.43; p<0.01); EC (t(16)=18.07; p<0.01)), such development-related increases did not occur in rats exposed to stressors during juvenility in the CA1 (t(16)=1.35; NS), DG (t(16)=1.27; NS), and EC (t(16)=0.64; NS); whereas in the BLA a significant decrease was observed (t(16)=3.02; p<0.05).
Effects of exposure to stressors during juvenility on development-related alterations in the expression levels of totalPSA-NCAM
Figure 5 depicts the effects of exposure to stressors during juvenility and the effects of development on totalPSA-NCAM levels in each of the monitored limbic regions. A two-way ANOVA for exposure to stressors during juvenility and for development indicated that in each of these regions totalPSA-NCAM expression levels were significantly affected by exposure to stressors during juvenility (BLA (F(1,32)=887.23; p<0.01); CA1 (F(1,32)=1059.90; p<0.01); DG (F(1,32)=514.68; p<0.01); EC (F(1,32)=42.19; p<0.01)) and by development (BLA (F(1,32)=617.40; p<0.01); CA1 (F(1,32)=352.73; p<0.01); DG (F(1,32)=595.31; p<0.01); EC (F(1,32)=28.83; p<0.01)). The interaction between juvenile stress and development was also significant for totalPSA-NCAM in the BLA (F(1,32)=5.12; p<0.05), DG (F(1,32)=9.54; p<0.01), and EC (F(1,32)=6.80; p<0.05), though not in the CA1 (F(1,32)=1.54; NS).
The effect of exposure to stressors during juvenility and the effect of development on totalPSA-NCAM levels across the limbic system: a two-way ANOVA indicated that, in each of the monitored regions (the BLA, the CA1 and DG regions of the hippocampus, and the EC, totalPSA-NCAM levels were significantly affected by exposure to stressors during juvenility and by development. The interaction effect between exposure to stressors during juvenility and development was significant in the BLA, DG, and EC regions, but not in the CA1. The insets depict representative bands of PSA-NCAM isoforms across groups (juvenile naive, J-N; juvenile-stressed, J-S; adult naive rats, A-N; and adult juvenile-stressed rats, AJ-S) and in the examined limbic regions: BLA, CA1, DG, and EC. At juvenility, totalPSA-NCAM levels were significantly higher in J-S rats (n=8) than in J-N rats (n=8) throughout all four limbic regions: BLA, CA1, DG, and EC. Similar significant differences were evident in adulthood, with increased totalPSA-NCAM expression levels in A-JS rats (n=10) compared with A-N rats (n=10) in all examined limbic regions. **significantly different from age-matched naive rats, p<0.01. *significantly different from age-matched naive rats, p<0.05. Among naive rats, totalPSA-NCAM levels decreased significantly during maturation from juvenility to adulthood in all four limbic regions: BLA, CA1, DG, and EC. In juvenile-stressed rats, totalPSA-NCAM levels decreased significantly during maturation from juvenility to adulthood in the BLA, CA1, and DG regions, but not in the EC. ##significantly different from juvenile rats of the same group, p<0.01.
Examining the effect of stress on totalPSA-NCAM expression levels in each age group
Additional t-tests with Bonferroni corrections compared totalPSA-NCAM levels between juvenile stress-exposed and naive rats during juvenility and adulthood. These comparisons indicated that, during juvenility, totalPSA-NCAM levels were significantly higher in juvenile-stressed rats (J-S, n=8) than in naive rats (J-N, n=8) throughout the limbic system (BLA (t(14)=12.60; p<0.01); CA1 (t(14)=16.45; p<0.01); DG (t(14)=12.46; p<0.01); EC (t(14)=2.60; p<0.05)). In adulthood, similarly increased totalPSA-NCAM levels were also evident in adult juvenile-stressed rats (A-JS, n=10) as compared with adult naive rats (A-N, n=10) throughout the limbic system (BLA (t(18)=73.15; p<0.01); CA1 (t(18)=42.87; p<0.01); DG (t(18)=27.76; p<0.01); EC (t(18)=6.84; p<0.05)).
Examining the effect of development on the totalPSA-NCAM expression levels among juvenile-stressed vs naive rats
Further t-tests with Bonferroni corrections were conducted to compare totalPSA-NCAM levels between juvenility and adulthood in rats exposed to stressors during juvenility and in naive rats. These comparisons indicated that during maturation from juvenility into adulthood totalPSA-NCAM levels decreased significantly in naive rats (J-N, n=8 vs A-N, n=10) in the BLA (t(16)=36.91; p<0.01); CA1 (t(16)=19.99; p<0.01); DG (t(16)=14.98; p<0.01); and EC (t(16)=7.17; p<0.01), as well as in rats exposed to stressors during juvenility (J-S, n=8 vs A-JS, n=10) in the BLA (t(16)=12.14; p<0.01), CA1 (t(16)=11.14; p<0.01), and DG (t(16)=19.55; p<0.01), though not in the EC (t(16)=1.66; NS). However, as indicated by the previous t-test, despite the significant development-related decrease in totalPSA-NCAM levels, totalPSA-NCAM expression was still significantly higher in adult juvenile-stressed rats than in adult naive rats.
Effects of Exposure to Stressors during Juvenility on Development-Related Changes in the Relative Expression of NCAM and Its Polysialylated Form PSA-NCAM
The developmental dynamics of NCAM and PSA-NCAM levels are opposite and complementary, with high levels of PSA-NCAM and relatively low levels of NCAM in early postnatal development, and the opposite in adulthood (Rønn et al, 1998). Chronic stress protocols in adult rats were also found to have opposite effects on the expression of NCAM and PSA-NCAM, decreasing the relative expression of NCAM, but increasing the relative expression of PSA-NCAM (Sandi, 2004).
We calculated the ratio of expression of totalPSA-NCAM to totalNCAM (totalPSA-NCAM/(totalNCAM+totalPSA-NCAM)) and examined stress-induced alterations to this ratio.
Figure 6 depicts the effects of exposure to stressors during juvenility and of development on the totalPSA-NCAM to totalNCAM expression ratio. As detailed below, a two-way ANOVA for exposure to stressors during juvenility and for development in each of the examined brain regions revealed a uniform pattern of effects on this ratio for exposure to stressors during juvenility, development, and for their interaction across all regions.
Effect of exposure to stressors during juvenility and effect of development on the totalPSA-NCAM to totalNCAM expression ratio across the limbic system: a two-way ANOVA for exposure to stressors during juvenility and development in each brain region indicated that the relative expression of totalPSA-NCAM to totalNCAM (totalPSA-NCAM/(totalPSA-NCAM+totalNCAM)) was affected by exposure to stressors during juvenility in a uniform manner across all tested limbic regions. A similar uniform pattern of effects was evident for development and for the interaction between exposure to stressors during juvenility and development. Comparisons of juvenile naive rats (J-N: n=8) with juvenile-stressed rats (J-S; n=8) revealed a mild yet significant stress-induced increase in the expression ratio of totalPSA-NCAM to totalNCAM soon after the exposure to stressors during juvenility in the BLA and in the CA1 region of the hippocampus, but not in the DG region of the hippocampus and EC. By contrast, in adulthood (adult naive (A-N); n=10; adult juvenile-stressed (A-JS); n=10) a significant and substantial juvenile stress-induced increase in the totalPSA-NCAM to totalNCAM expression ratio was evident in all four limbic regions: BLA, CA1, DG, and EC. **Significantly different from age matched naive rats, p<0.01. Exposure to stressors during juvenility disrupted the normative developmental decrease in the totalPSA-NCAM to totalNCAM expression ratio from juvenility to adulthood. A significant and substantial developmental decrease in the totalPSA-NCAM to totalNCAM expression ratio was evident only among naive rats (J-N, n=8; A-N, n=10) in all examined regions: BLA, CA1, DG, and EC. In contrast, among juvenile-stressed rats (J-S, n=8; A-JS, n=10), no differences in the totalPSA-NCAM to totalNCAM expression ratio were evident in the BLA, CA1 or EC, whereas the mild, but significant, decrease found only in the DG was incomparable with that found in naive rats. ##significantly different from juvenile rats of the same group, p<0.01.
Examining the effects of juvenile stress exposure and development by brain region
Exposure to stressors during juvenility significantly affected the totalPSA-NCAM to totalNCAM expression ratio in each of the examined regions (BLA (F(1,32)=857.19; p<0.01); CA1 (F(1,32)=367.70; p<0.01); DG (F(1,32)=589.94; p<0.01); EC (F(1,32)=87.09; p<0.01)). Similarly, a significant developmental effect was found in each region (BLA (F(1,32)=637.24; p<0.01); CA1 (F(1,32)=131.41; p<0.01); DG (F(1,32)=842.90; p<0.01); EC (F(1,32)=147.29; p<0.01)), with significant interactions between exposure to stressors during juvenility and development in all examined regions (BLA (F(1,32)=570.60; p<0.01); CA1 (F(1,32)=127.31; p<0.05); DG (F(1,32)=498.63; p<0.01); EC (F(1,32)=99.35; p<0.01)).
Examining the effect of development on the totalPSA-NCAM to totalNCAM expression ratios among juvenile-stressed vs naive rats
Additional t-tests with Bonferroni corrections compared the totalPSA-NCAM to totalNCAM expression ratios of juvenile stress-exposed and naive rats during juvenility and adulthood. These comparisons indicated that exposure to stressors during juvenility hindered the normal course of development, which manifests as a decrease in the totalPSA-NCAM to totalNCAM expression ratio from juvenility to adulthood.
A significant developmental decrease in the totalPSA-NCAM to totalNCAM expression ratio in all limbic regions was evident only in naive rats (BLA (t(16)=32.08; p<0.01); CA1 (t(16)=17.60; p<0.01); DG (t(16)=36.62; p<0.01); EC (t(16)=18.44; p<0.01)). However, in juvenile-stressed rats, a significant decrease in the totalPSA-NCAM to totalNCAM expression ratio was not evident in the BLA (t(16)=1.05; NS), CA1 (t(16)=0.109; NS), or EC (t(16)=1.33; NS). Although in the DG a mild yet significant decrease was found (t(16)=4.56; p<0.01), its magnitude was incomparable with that found in naive rats.
Examining the effect of exposure to stressors during juvenility on the totalPSA-NCAM to totalNCAM expression ratios among juvenile vs adult rats
Further t-tests with Bonferroni corrections compared the effect of exposure to stressors during juvenility on the totalPSA-NCAM to totalNCAM expression ratio as measured soon after the exposure during juvenility and in adulthood. A mild yet significant stress-induced increase in this ratio was found soon after exposure to stressors in the BLA (t(16)=3.59; p<0.01) and CA1 (t(16)=4.39; p<0.01) but not in the DG (t(16)=1.22; NS) or EC (t(16)=0.41; NS). However, in adulthood, a significant and substantial juvenile stress-induced increase in the totalPSA-NCAM to totalNCAM expression ratio was evident in all examined regions (BLA (t(16)=40.63; p<0.01); CA1 (t(16)=28.37; p<0.01); DG (t(16)=37.26; p<0.01); EC (t(16)=15.08; p<0.01)).
DISCUSSION
NCAMs mediate cell–cell interactions and are recognized as key players in activity-dependent synaptic remodeling, memory storage (Welzl and Stork, 2003), and developmental events (Dey et al, 1999). Exposure to stressors was found to affect the functioning of NCAMs in adult rats. These alterations were suggested to be associated with heightened anxiety, impaired cognitive and neural function, and compromised synaptic plasticity processes (Theodosis et al, 1999; Sandi et al, 2001, 2003; Sandi, 2004). The current study set out to characterize the relationship between the effects that exposure to stressors during juvenility imposes on emotional responses in adulthood, as manifested in altered behaviors under stressful conditions, and on development-related alterations in the expression of NCAM and its posttranslationally polysialylated form, PSA-NCAM. The current study also reconfirmed previously reported findings (Avital and Richter-Levin, 2005; Tsoory and Richter-Levin, 2006; Tsoory et al, 2007) that exposure to stressors during juvenility alters stress-coping responses in adulthood. Furthermore, these behavioral alterations were coupled with changes in the protein-expression levels of NCAM and PSA-NCAM, with these changes appearing shortly after exposure to stressors during juvenility and lasting into adulthood.
Exposure to Stress during Juvenility Alters Emotional Responses in Adulthood
As previously demonstrated, exposure to stressors during juvenility results in altered emotional responses in adulthood, that is, a marked reduction in novel-setting exploration, impaired avoidance learning, and more escape failures in the two-way shuttle avoidance task (Tsoory and Richter-Levin, 2006; Tsoory et al, 2007) (Figure 3). Reduction in novel-setting exploration by rats following exposure to stressors is a commonly used and pharmacologically validated index for heightened anxiety, which is highly correlated with other accepted behavioral and physiological anxiety indices (Kalynchuk et al, 1997; Ramos and Mormede, 1998; Campbell et al, 2003; Gregus et al, 2005).
As learning and performance in the two-way shuttle avoidance task are dependent on the hippocampus (Schwegler et al, 1981; Becker et al, 1997) and the amygdalae (Savonenko et al, 2003), the impaired avoidance learning observed in adult juvenile-stressed rats may also indicate compromised emotional responses, possibly mediated by altered functioning of these neurocircuits. Poor two-way shuttle avoidance performance was observed following both negligible and high doses of injected CORT (Kademian et al, 2005). Selective breeding based on ‘high/low-avoidance’ performance was suggested to relate to differences in ‘emotional’ factors (state/trait anxiety) that influenced performance (Brush, 2003). For example, the ‘high-avoidance’ and ‘low-avoidance’ Hatano rat strains did not differ in locomotion activity (Ohta et al, 1999), but the ‘low-avoidance’ strain had a higher adrenocorticotropin (ACTH)-induced adrenal CORT release response than the ‘high-avoidance’ strain (Asai et al, 2004). The increased rates of escape failure in the shuttle box that we found among adult juvenilestressed rats (Figure 3c) may also imply an emotional disruption. Such increases in escape failures were suggested to correspond to learned helplessness, representing, in animals, depressive symptoms of nonresponsiveness (Pryce et al, 2005). Increased rates of escape failure follow exposure to inescapable/uncontrollable stressors (Russig et al, 2003; Rüedi-Bettschen et al, 2005); are more frequent among adult rats that were exposed to stressors during the perinatal period (Rüedi-Bettschen et al, 2005); and may be attenuated by antidepressants (Weiss and Kilts, 1998; Chen et al, 2001; Vollmayr and Henn, 2001; Rüedi-Bettschen et al, 2004).
The behavioral effects of the current study support previous findings indicating that in the course of the neural development of the rat juvenility is a stress-sensitive period in which even a rather short-term period of exposure to stressors results in persistent behavioral disruptions. Exposure to stressors during juvenility augmented the effect of exposure to stressors in adulthood, reducing exploration in the open field whereas increasing startle responses and affecting water maze performance (Avital and Richter-Levin, 2005). Exposure to different short-term stressors during juvenility resulted in impaired coping responses when faced with stressors in adulthood, resembling both anxious and depressive symptoms (Tsoory et al, 2007). These ‘juvenile stress’ (27–29 days of age) effects were found to be stronger than the effects of exposure to the same stressors during mid-adolescence (33–35 days of age) (Tsoory and Richter-Levin, 2006).
Exposure to stressors during juvenility alters development-related changes in totalNCAM expression
In the current study, exposure to stressors during juvenility also altered the expression levels of NCAM and PSA-NCAM throughout the monitored limbic brain regions shortly after the exposure and also in adulthood.
The expression of totalNCAM did not appear affected by juvenile exposure to stress shortly after that exposure, at the age of 33 days. However, in adulthood, at 9 weeks of age, decreased expression of totalNCAM was evident among juvenile-stressed rats (Figure 4).
During juvenility, NCAM expression levels appear low compared with adulthood. They rise in the course of neural development as depolysialylation of PSA-NCAM commences and NCAMs are expressed on the cell surface, forming cell-cell adhesions (Rønn et al, 1998). This normative developmental increase was evident in our study in the comparison of naive juvenile with naive adult rats in terms of totalNCAM expression levels (Figure 4). However, exposure to stressors during juvenility seems to alter the normative development-related increase in NCAM expression. The low levels of totalNCAM observed at 33 days of age did not increase significantly during the maturation process in juvenile-stressed rats as they did in controls. At the age of 9 weeks, adult juvenile-stressed rats exhibited totalNCAM levels that were significantly lower than those of the controls in all four brain regions: BLA, CA1, DG, and EC (Figure 4).
Several studies on adult rats have associated chronic stress protocols known to impair cognitive capacities and alter neural functioning with lower expression levels of various NCAM isoforms, mainly within the hippocampus (Sandi et al, 2001; Touyarot and Sandi, 2002; Touyarot et al, 2004; Venero et al, 2002). In a parallel vein, interference with hippocampal NCAM functioning by conditional ablation of the NCAM gene in adult mutant mice (NCAMff+) yielded mimicry of the deleterious effect of stress exposure on both long-term potentiation (LTP) and long-term depression in the CA1, as well as on spatial orientation (Bukalo et al, 2004). Likewise, chronic variable stress protocols applied in the prenatal or postweaning period induced a profound downregulation in NCAM expression levels in the hippocampus and cortex, coupled with cognitive impairments and a reduction in the expression of the synaptic marker synaptophysin and BDNF (Koo et al, 2003).
Originally, NCAM-null mutant mice were reported to develop normally, with only minor anatomic aberrations in the olfactory bulbs and hippocampal mossy fiber system (Cremer et al, 1994). Later studies reported that, as adults, these NCAM-null mutants showed a reduction in thorny excrescences in CA3 pyramidal cells, as well as alterations in mossy fiber growth and fasciculation (Cremer et al, 1997; Cremer et al, 1998). As adults, the exploratory behavior of these null mutant mice, their spatial learning, fear conditioning, and LTP were inferior to those of the wild-type, and the mice were characterized by increased aggressiveness as well as alterations in emotional/motivational behavior and hyperresponsiveness of the serotonergic system (Muller et al, 1996; Stork et al, 1997, 1999, 2000). Interestingly, in these mutants, ‘anxious’ behaviors, but not learning, could be rescued through a transgenic manipulation causing reexpression of the NCAM-180 kDa isoform (Stork et al, 2000).
Several studies have indicated that glucocorticoid receptor activation may repress transcription and/or splicing of NCAM (Colwell et al, 1992; Quandt et al, 1995; De Bosscher et al, 1997; Simpson and Morris, 2000; Datson et al, 2001; Karin and Chang, 2001; Takeuchi and Fukunaga, 2003; Sandi, 2004).
As after exposure to stressors, plasma CORT concentration levels in juvenile rats return to baseline levels at half the pace observed in adult rats (Romeo et al, 2004); the role of these glucocorticoids in the regulation of NCAM gene transcription should be considered. Adult juvenile stress rats exhibit an array of heightened anxiety indices (Avital and Richter-Levin, 2005; Tsoory and Richter-Levin, 2006, Tsoory et al, 2007), suggesting sensitized stress-response mechanisms which may include glucocorticoid receptor activation.
The fact that shortly after the exposure to the stressors during juvenility no significant decrease in totalNCAM expression was evident (Figure 4) may be due to a floor effect. That is, the normative low levels of NCAM at this age may be such that they cannot be downregulated further.
Exposure to stressors during juvenility alters development-related changes in totalPSA-NCAM expression
Exposure to stressors during juvenility altered PSA-NCAM levels in the BLA, CA1, DG, and EC. Increased totalPSA-NCAM expression was observed shortly after exposure to the stressors during juvenility as well as in adulthood (Figure 5). High levels of PSA-NCAM are expressed during early development and decrease as the animal matures (Doyle et al, 1992b; Rønn et al, 2000). In our study, this development-related decrease was evident when naive juvenile and adult rats were compared for their totalPSA-NCAM expression levels (Figure 5). A similar development-related decrease in totalPSA-NCAM expression was evident in juvenile-stressed rats, but because exposure to the stressors during juvenility significantly elevated PSA-NCAM expression, it was still significantly higher in adult juvenile-stressed rats than in naive adult rats. This may suggest that the stressors applied did not arrest this developmental process, but shifted its set point upward.
Several studies on adult rats have associated chronic stress exposure or repeated glucocorticoid administration-induced cognitive impairments with altered synaptic plasticity and increased levels of PSA-NCAM in different hippocampal subregions (for a review, see Sandi, 2004). However, acute restraint procedures, which failed to induce hippocampal structural alteration, also failed to increase polysialylation of NCAM (Pham et al, 2003). To the best of our knowledge, our study presents the first evidence of a persistent increase in expression levels of totalPSA-NCAM following a nonchronic stress procedure. These effects may be related to juvenile rats having an enhanced stress response (Romeo et al, 2004), which may have interacted with a limbic system that was underdeveloped and more susceptible to the effects of stress, and set into motion a PSA-NCAM counterreaction. The de-adhesive properties of PSA-NCAMs have been suggested to counteract the stress-induced excess glutamatergic input (Hoffman et al, 1997; Rutishauser, 1998; Sandi, 2004) and/or the reduction in BDNF that follows chronic stress protocols (Smith et al, 1995). As polysialylation of NCAM is critical for neurogenesis (Seki and Arai, 1993; Franceschini et al, 2001) and for synaptic plasticity (Muller et al, 1996), increased PSA-NCAM expression levels were suggested to represent a compensatory response to the deleterious structural effects of stress (Sandi et al, 2001).
Exposure to stressors during juvenility alters development-related changes in the dynamic equilibrium between totalNCAM and totalPSA-NCAM
The equilibrium between the expression levels of PSA-NCAM and NCAM changes gradually from high levels of PSA-NCAM and low levels of NCAM in early postnatal development to the opposite in adulthood (Doyle et al, 1992b; Rønn et al, 1998; Rønn et al, 2000). These changes are regulated, on the one hand, by varying dynamics in the activity of ST8SIAIV/PST, a prominent polysialyltransferase (PST) in the postnatal brain, which binds the PSA-branched moieties to NCAM, and on the other hand, by a counteracting depolysialylation process which reduces expression of PSA-NCAM (Doyle et al, 1992b; Rønn et al, 2000).
Our data indicate that, soon after exposure to stressors during juvenility, totalPSA-NCAM levels increased (Figure 5), whereas totalNCAM levels were not affected (Figure 4). However, adult juvenile-stressed rats exhibited a relatively reduced level of totalNCAM (Figure 4), coupled with an increased level of totalPSA-NCAM, even though a significant development-related decrease in totalPSA-NCAM levels did occur with maturation, as in naive rats (Figure 5). This may imply that exposure to stressors during juvenility induced an enhanced and maybe persistent polysialylation action, possibly via continuous transcription of PST, producing increased levels of totalPSA-NCAM which, in the long run, may have overshadowed the effects of depolysialylation of totalPSA-NCAM, resulting in relatively lower levels of totalNCAM in adulthood. An immature equilibrium between these NCAMs may underlie faulty activity-dependent synaptic remodeling processes in the adult rat, rendering it susceptible to the effects of stress.
Summary and concluding remarks
Taken together, our data demonstrate altered emotional responses in adulthood following exposure to stressors during juvenility which were coupled with altered development-related changes in the expression of totalNCAM and its posttranslationally modified form totalPSA-NCAM. They strongly support the possibility of a role for altered functioning of these NCAMs, and possibly others, in generating experience-induced predispositions to stress-related psychopathologies.
It is noteworthy that the effects of development, exposure to stressors during juvenility, and their interaction on totalNCAM and totalPSA-NCAM expression appeared to be similar in all the examined limbic tissues, the BLA, the CA1 and DG subregions of the hippocampus, and the EC. These regions are well recognized for their pivotal role and interactive function in modulating emotional faculties as well as learning processes (Richter-Levin, 2004). Therefore, it would be interesting to evaluate the effects that exposure to stressors during juvenility may have on learning nonaversive tasks in adulthood and to correlate these effects with those it may impose on the expression of totalNCAM and totalPSA-NCAM. Furthermore, because, during the adolescent period, substantial maturation processes occur in several brain mechanisms in different regions, including the prefrontal cortex (Spear, 2000), and considering the apparent nonspecificity of the effects regarding brain regions in the current study, it is tempting to speculate that exposure to stressors during juvenility may also affect development-related alterations in the expression of these and other NCAMs in other brain regions.
Modulations to the functioning of NCAMs are under investigation for their potential therapeutic effects in brain repair (Webb et al, 2001; Nguyen et al, 2003) and in neural and cognitive disorders (Berezin and Bock, 2004). Our ‘juvenile stress’ model, along with totalNCAM and totalPSA-NCAM expression assessments, may be utilized as an effective animal model for testing the antidepressant and/or anxiety-relieving competence of new treatments that modulate the regulation of NCAMs.
References
Arami S, Jucker M, Schachner M, Welzl H (1996). The effect of continuous intraventricular infusion of L1 and NCAM antibodies on spatial learning in rats. Behav Brain Res 1–2: 81–87.
Asai S, Ohta R, Shirota M, Watanabe G, Taya K (2004). Differential responses of the hypothalamo-pituitary-adrenocortical axis to acute restraint stress in Hatano high- and low-avoidance rats. J Endocrinol 3: 515–520.
Avital A, Ram E, Maayan R, Weizman A, Richter-Levin G (2006). Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bull 6: 419–424.
Avital A, Richter-Levin G (2005). Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol 2: 163–173.
Avital A, Richter-Levin G, Leschiner S, Spanier I, Veenman L, Weizman A et al (2001). Acute and repeated swim stress effects on peripheral benzodiazepine receptors in the rat hippocampus, adrenal, and kidney. Neuropsychopharmacology 5: 669–678.
Becker A, Letzel K, Letzel U, Grecksch G (1997). Kindling of the dorsal and the ventral hippocampus: effects on learning performance in rats. Physiol Behav 6: 1265–1271.
Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M (1996). The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res 2: 143–152.
Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z (2005). Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res 161: 45–59.
Berezin V, Bock E (2004). NCAM mimetic peptides: Pharmacological and therapeutic potential. J Mol Neurosci 1-2: 33–39.
Brush FR (2003). Selection for differences in avoidance learning: the Syracuse strains differ in anxiety, not learning ability. Behav Genet 6: 677–696.
Bukalo O, Fentrop N, Lee AY, Salmen B, Law JW, Wotjak CT et al (2004). Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci 7: 1565–1577.
Campbell T, Lin S, DeVries C, Lambert K (2003). Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav 3: 495–504.
Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS (2001). Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry 9: 753–762.
Colwell G, Li B, Forrest D, Brackenbury R (1992). Conserved regulatory elements in the promoter region of the N-CAM gene. Genomics 4: 875–882.
Cordero MI, Rodriguez JJ, Davies HA, Peddie CJ, Sandi C, Stewart MG (2005). Chronic restraint stress down-regulates amygdaloid expression of polysialylated neural cell adhesion molecule. Neuroscience 4: 903–910.
Covault J (1989). Molecular biology of cell adhesion in neural development. In: Glover DM, Hames BD (eds). Molecular Neurobiology. Oxford Univ Press: New York. pp 143–200.
Cremer H, Chazal G, Carleton A, Goridis C, Vincent JD, Lledo PM (1998). Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Acad Sci USA 22: 13242–13247.
Cremer H, Chazal G, Goridis C, Represa A (1997). NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci 5: 323–335.
Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J et al (1994). Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 6462: 455–459.
Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E (2001). Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci 4: 675–689.
De Bosscher K, Schmitz ML, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G (1997). Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA 25: 13504–13509.
Degroot A, Wade M, Salhoff C, Davis RJ, Tzavara ET, Nomikos GG (2004). Exposure to an elevated platform increases plasma corticosterone and hippocampal acetylcholine in the rat: reversal by chlordiazepoxide. Eur J Pharmacol 1-3: 103–109.
Dey PM, Gochfeld M, Reuhl KR (1999). Developmental methylmercury administration alters cerebellar PSA-NCAM expression and Golgi sialyltransferase activity. Brain Res 2: 139–151.
Doherty P, Walsh FS (1991). The contrasting roles of N-CAM and N-cadherin as neurite outgrowth-promoting molecules. J Cell Sci Suppl 15: 13–21.
Doyle E, Nolan PM, Bell R, Regan CM (1992a). Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J Neurosci Res 3: 513–523.
Doyle E, Nolan PM, Bell R, Regan CM (1992b). Intraventricular infusions of anti-neural cell adhesion molecules in a discrete posttraining period impair consolidation of a passive avoidance response in the rat. J Neurochem 4: 1570–1573.
Ebner K, Rupniak NM, Saria A, Singewald N (2004). Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci USA 12: 4280–4285.
Edelman GM (1984). Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu Rev Neurosci 7: 339–377.
Einon DF, Morgan MJ (1977). A critical period for social isolation in the rat. Dev Psychobiol 2: 123–132.
Foley AG, Hartz BP, Gallagher HC, Ronn LC, Berezin V, Bock E et al (2000). A synthetic peptide ligand of neural cell adhesion molecule (NCAM) IgI domain prevents NCAM internalization and disrupts passive avoidance learning. J Neurochem 6: 2607–2613.
Franceschini I, Angata K, Ong E, Hong A, Doherty P, Fukuda M (2001). Polysialyltransferase ST8Sia II (STX) polysialylates all of the major isoforms of NCAM and facilitates neurite outgrowth. Glycobiology 3: 231–239.
Friedlander DR, Brackenbury R, Edelman GM (1985). Conversion of embryonic form to adult forms of N-CAM in vitro: results from de novo synthesis of adult forms. J Cell Biol 2: 412–419.
Garcia-Vallejo P, Gomez FM, Infante C, Ginestal E, Giralt MT (1998). Chronic variable stress induces supersensitivity of central alpha2-adrenoceptors which modulate the jaw-opening reflex in the rat. Brain Res 1-2: 72–77.
Gregus A, Wintink AJ, Davis AC, Kalynchuk LE (2005). Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 1: 105–114.
Hall FS, Sundstrom JM, Lerner J, Pert A (2001). Enhanced corticosterone release after a modified forced swim test in Fawn hooded rats is independent of rearing experience. Pharmacol Biochem Behav 3-4: 629–634.
Heim C, Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 12: 1023–1039.
Heim C, Plotsky PM, Nemeroff CB (2004). Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 4: 641–648.
Hoffman KB, Kessler M, Lynch G (1997). Sialic acid residues indirectly modulate the binding properties of AMPA-type glutamate receptors. Brain Res 2: 309–314.
Hoffman S, Sorkin BC, White PC, Brackenbury R, Mailhammer R, Rutishauser U et al (1982). Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem 13: 7720–7729.
Horovitz O, Jacobson-Pick S, Tsoory MM, Richter-Levin G (2007). Exposing rats to juvenile stress affect body weight and open field indices both soon after the exposure and in adulthood, but in opposite directions. Neural Plasticity—Abstracts of the 15th Annual Meeting of the Israel Society for Neurosciences, Eilat, Israel, 3–5 December, 2000, Vol 1, p 46.
Kademian SM, Bignante AE, Lardone P, McEwen BS, Volosin M (2005). Biphasic effects of adrenal steroids on learned helplessness behavior induced by inescapable shock. Neuropsychopharmacology 1: 58–66.
Kalynchuk LE, Pinel JP, Treit D, Kippin TE (1997). Changes in emotional behavior produced by long-term amygdala kindling in rats. Biol Psychiatry 4: 438–451.
Karin M, Chang L (2001). AP-1—glucocorticoid receptor crosstalk taken to a higher level. J Endocrinol 3: 447–451.
Keilhauer G, Faissner A, Schachner M (1985). Differential inhibition of neurone–neurone, neurone–astrocyte and astrocyte–astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature 6030: 728–730.
Kiss JZ, Troncoso E, Djebbara Z, Vutskits L, Muller D (2001). The role of neural cell adhesion molecules in plasticity and repair. Brain Res Brain Res Rev 2-3: 175–184.
Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC et al (2003). The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. FASEB J 11: 1556–1558.
Li HY, Sawchenko PE (1998). Hypothalamic effector neurons and extended circuitries activated in ‘neurogenic’ stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol 2: 244–266.
Lüthi A, Laurent JP, Figurov A, Muller D, Schachner M (1994). Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature 6508: 777–779.
Maroun M, Richter-Levin G (2003). Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 11: 4406–4409.
Martijena ID, Rodriguez Manzanares PA, Lacerra C, Molina VA (2002). Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse 2: 86–94.
Maslova LN, Bulygina VV, Markel AL (2002). Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology 5: 549–561.
McEwen BS (1999). Stress and hippocampal plasticity. Ann Rev Neurosci 22: 105–122.
McGivern RF, Henschel D, Hutcheson M, Pangburn T (1996). Sex difference in daily water consumption of rats: effect of housing and hormones. Physiol Behav 4-5: 653–658.
Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T (2001). Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 26: 443–459.
Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V et al (1996). PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 3: 413–422.
Nacher J, Pham K, Gil-Fernandez V, McEwen BS (2004). Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience 2: 503–509.
Nemeroff CB (2004). Neurobiological consequences of childhood trauma. J Clin Psychiatry 65 (Suppl 1): 18–28.
Nguyen L, Rigo JM, Malgrange B, Moonen G, Belachew S (2003). Untangling the functional potential of PSA-NCAM-expressing cells in CNS development and brain repair strategies. Curr Med Chem 20: 2185–2196.
Nybroe O, Bock E (1990). Structure and function of the neural cell adhesion molecules NCAM and L1. Adv Exp Med Biol 265: 185–196.
O'Connell AW, Fox GB, Barry T, Murphy KJ, Fichera G, Foley AG et al (1997). Spatial learning activates neural cell adhesion molecule polysialylation in a corticohippocampal pathway within the medial temporal lobe. J Neurochem 6: 2538–2546.
Ohta R, Shirota M, Adachi T, Tohei A, Taya K (1999). Plasma ACTH levels during early, two-way avoidance acquisition in high- and low-avoidance rats (Hatano strains). Behav Genet 2: 137–144.
Pace TW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL (2005). Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci 7: 1679–1690.
Passerin AM, Cano G, Rabin BS, Delano BA, Napier JL, Sved AF (2000). Role of locus coeruleus in foot shock-evoked Fos expression in rat brain. Neuroscience 4: 1071–1082.
Paxinos G, Watson C (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, USA.
Pezzone MA, Lee WS, Hoffman GE, Pezzone KM, Rabin BS (1993). Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain Res 2: 310–318.
Pezzone MA, Lee WS, Hoffman GE, Rabin BS (1992). Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res 1: 41–50.
Pham K, Nacher J, Hof PR, McEwen BS (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 4: 879–886.
Prieto M, Gomez FM, Teresa Giralt M (2003). Effects of acute, repeated and chronic variable stress on in vivo tyrosine hydroxylase activity and on alpha(2)-adrenoceptor sensitivity in the rat brain. Stress 4: 281–287.
Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B et al (2005). Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neurosci Biobehav Rev 4-5: 649–674.
Quandt K, Frech K, Karas H, Wingender E, Werner T (1995). MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23: 4878–4884.
Ramos A, Mormede P (1998). Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 1: 33–57.
Rassnick S, Hoffman GE, Rabin BS, Sved AF (1998). Injection of corticotropin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience 1: 259–268.
Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J (2003). Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 28: 207–227.
Richter-Levin G (2004). The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist 1: 31–39.
Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS (2004). Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology 3: 125–132.
Rønn LC, Berezin V, Bock E (2000). The neural cell adhesion molecule in synaptic plasticity and ageing. Int J Dev Neurosci 2-3: 193–199.
Rønn LC, Hartz BP, Bock E (1998). The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol 7-8: 853–864.
Rose SP (1995). Glycoproteins and memory formation. Behav Brain Res 1-2: 73–78.
Rüedi-Bettschen D, Feldon J, Pryce CR (2004). The impaired coping induced by early deprivation is reversed by chronic fluoxetine treatment in adult Fischer rats. Behav Pharmacol 5-6: 413–421.
Rüedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR (2005). Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res 2: 297–310.
Rusakov DA, Davies HA, Krivko IM, Stewart MG, Schachner M (1994). Training in chicks alters PSA-N-CAM distribution in forebrain cell membranes. Neuroreport 18: 2469–2473.
Russig H, Pezze MA, Nanz-Bahr NI, Pryce CR, Feldon J, Murphy CA (2003). Amphetamine withdrawal does not produce a depressive-like state in rats as measured by three behavioral tests. Behav Pharmacol 1: 1–18.
Rutishauser U (1989). Neural cell-to-cell adhesion and recognition. Curr Opin Cell Biol 5: 898–904.
Rutishauser U (1990). Neural cell adhesion molecule as a regulator of cell–cell interactions. Adv Exp Med Biol 265: 179–183.
Rutishauser U (1998). Polysialic acid at the cell surface: biophysics in service of cell interactions and tissue plasticity. J Cell Biochem 3: 304–312.
Rutishauser U, Jessell TM (1988). Cell adhesion molecules in vertebrate neural development. Physiol Rev 3: 819–857.
Rutishauser U, Landmesser L (1996). Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell–cell interactions. Trends Neurosci 10: 422–427.
Sanchez MM, Ladd CO, Plotsky PM (2001). Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 3: 419–449.
Sandi C (2004). Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci 12: 917–930.
Sandi C, Cordero MI, Merino JJ, Kruyt ND, Regan CM, Murphy KJ (2004). Neurobiological and endocrine correlates of individual differences in spatial learning ability. Learn Mem 3: 244–252.
Sandi C, Merino JJ, Cordero MI, Kruyt ND, Murphy KJ, Regan CM (2003). Modulation of hippocampal NCAM polysialylation and spatial memory consolidation by fear conditioning. Biol Psychiatry 6: 599–607.
Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C (2001). Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience 2: 329–339.
Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL et al (2006). PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs Adult trauma. Psychoneuroendocrinology 31: 501–509.
Savonenko A, Werka T, Nikolaev E, Zielinski K, Kaczmarek L (2003). Complex effects of NMDA receptor antagonist APV in the basolateral amygdala on acquisition of two-way avoidance reaction and long-term fear memory. Learn Mem 4: 293–303.
Schachner M (1997). Neural recognition molecules and synaptic plasticity. Curr Opin Cell Biol 5: 627–634.
Schwegler H, Lipp HP, Van der Loos H, Buselmaier W (1981). Individual hippocampal mossy fiber distribution in mice correlates with two-way avoidance performance. Science 4522: 817–819.
Seki T, Arai Y (1993). Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 6: 2351–2358.
Simpkiss JL, Devine DP (2003). Responses of the HPA axis after chronic variable stress: effects of novel and familiar stressors. Neuro endocrinology letters 1-2: 97–103.
Simpson CS, Morris BJ (2000). Regulation of neuronal cell adhesion molecule expression by NF-kappa B. J Biol Chem 22: 16879–16884.
Smith MA, Makino S, Kvetnansky R, Post RM (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 3 (Part 1): 1768–1777.
Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 4: 417–463.
Stork O, Welzl H, Cremer H, Schachner M (1997). Increased intermale aggression and neuroendocrine response in mice deficient for the neural cell adhesion molecule (NCAM). Eur J Neurosci 6: 1117–1125.
Stork O, Welzl H, Wolfer D, Schuster T, Mantei N, Stork S et al (2000). Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) null mutant mice through transgenic expression of NCAM180. Eur J Neurosci 9: 3291–3306.
Stork O, Welzl H, Wotjak CT, Hoyer D, Delling M, Cremer H et al (1999). Anxiety and increased 5-HT1A receptor response in NCAM null mutant mice. J Neurobiol 3: 343–355.
Takeuchi Y, Fukunaga K (2003). Differential regulation of NF-kappaB, SRE and CRE by dopamine D1 and D2 receptors in transfected NG108-15 cells. J Neurochem 3: 729–739.
Theodosis DT, Bonhomme R, Vitiello S, Rougon G, Poulain DA (1999). Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J Neurosci 23: 10228–10236.
Tiunova A, Anokhin KV, Schachner M, Rose SP (1998). Three time windows for amnestic effect of antibodies to cell adhesion molecule L1 in chicks. Neuroreport 7: 1645–1648.
Touyarot K, Sandi C (2002). Chronic restraint stress induces an isoform-specific regulation on the neural cell adhesion molecule in the hippocampus. Neural Plast 3: 147–159.
Touyarot K, Venero C, Sandi C (2004). Spatial learning impairment induced by chronic stress is related to individual differences in novelty reactivity: search for neurobiological correlates. Psychoneuroendocrinology 2: 290–305.
Tsoory M, Cohen H., Richter- Levin G (2004). ‘Juvenile’ stress as a risk factor for impaired coping behavior in adulthood—a rat model. Neural Plasticity—Abstracts of the 13th Annual Meeting of the Israel Society for Neurosciences, Eilat, Israel, 28–30 November, 2004, Vol 12, p 62.
Tsoory M, Cohen H, Richter-Levin G (2007). Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol 17: 245–256.
Tsoory M, Richter-Levin G (2006). Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolecent’ stress. Int J Neuropsychopharmacol 6: 713–728.
Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM (1999a). Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res 1-2: 133–142.
Van den Berg CL, Van Ree JM, Spruijt BM (1999b). Sequential analysis of juvenile isolation-induced decreased social behavior in the adult rat. Physiol Behav 4: 483–488.
Van den Berg CL, Van Ree JM, Spruijt BM (2000). Morphine attenuates the effects of juvenile isolation in rats. Neuropharmacology 6: 969–976.
Van den Berg CL, Van Ree JM, Spruijt BM, Kitchen I (1999c). Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. Euro J Neurosci 9: 3023–3032.
Vazquez DM (1998). Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 7: 663–700.
Venero C, Tilling T, Hermans-Borgmeyer I, Schmidt R, Schachner M, Sandi C (2002). Chronic stress induces opposite changes in the mRNA expression of the cell adhesion molecules NCAM and L1. Neuroscience 4: 1211–1219.
Vollmayr B, Henn FA (2001). Learned helplessness in the rat: improvements in validity and reliability. Brain Res Brain Res Protoc 1: 1–7.
Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA (2001). Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials 10: 1017–1028.
Weiss J, Kilts CD (1998). Animal models of depression and schizophrenia. In: Nemeroff CB, Schatzberg AF (eds). Textbook of Psychopharmacology. American Psychiatric Press: Washington, DC. pp 88–123.
Welzl H, Stork O (2003). Cell adhesion molecules: key players in memory consolidation? News Physiol Sci 18: 147–150.
Acknowledgements
We thank the reviewers for their knowledgeable remarks which helped us to improve the manuscript.
This work was supported by the EU's PROMEMORIA grant #512012 to GRL. Many thanks to Professor Rita Gerardy-Schahn of the Hannover Medical School for her invaluable advice and insights.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsoory, M., Guterman, A. & Richter-Levin, G. Exposure to Stressors during Juvenility Disrupts Development-Related Alterations in the PSA-NCAM to NCAM Expression Ratio: Potential Relevance for Mood and Anxiety Disorders. Neuropsychopharmacol 33, 378–393 (2008). https://doi.org/10.1038/sj.npp.1301397
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301397
Keywords
This article is cited by
-
Acute and long-term effects of adolescence stress exposure on rodent adult hippocampal neurogenesis, cognition, and behaviour
Molecular Psychiatry (2023)
-
Acute Sleep Deprivation-Induced Anxiety and Disruption of Hypothalamic Cell Survival and Plasticity: A Mechanistic Study of Protection by Butanol Extract of Tinospora cordifolia
Neurochemical Research (2022)
-
Butanol Extract of Tinospora cordifolia Alleviates Acute Sleep Deprivation-Induced Impairments in Cognitive Functions and Neuromuscular Coordination in Middle-Aged Female Rats
NeuroMolecular Medicine (2022)
-
Withania somnifera (L.) Dunal ameliorates neurodegeneration and cognitive impairments associated with systemic inflammation
BMC Complementary and Alternative Medicine (2019)
-
Fluoxetine treatment is effective in a rat model of childhood-induced post-traumatic stress disorder
Translational Psychiatry (2017)