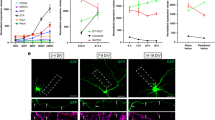
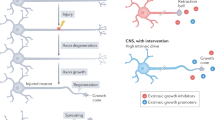
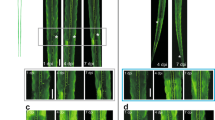
Key Points
-
Before a cut axon can attempt to grow, it first has to assemble a new growth cone at its tip.
-
The mechanism leading to the assembly of a new growth cone includes the following events: membrane seal formation; calcium influx; activation of kinases, phosphatases and proteases; local restructuring of the cytoskeleton; accumulation of Golgi-derived vesicles; and fusion of such vesicles with the plasma membrane. These events are supported by concurrent local protein translation and anterograde transport of proteins that are in transit in the axon, but are probably not supported by new protein synthesis in the cell body.
-
During the formation of a frustrated growth cone or end bulb, vesicles that accumulate at the cut end of an axon fail to fuse with the plasma membrane, and the microtubules point their tips in a retrograde direction. End bulb formation can result from the failure of any one of the events described above.
-
In the future, it may be possible to prevent the failure of growth cone regeneration by stabilizing microtubules with nanomolar concentrations of taxol; by enhancing or enabling the transport of protein translation machinery (for example, mRNA and ribosomes) from the cell body to the site of injury through changing the 'filtering' properties of the axon's initial segment; or by facilitating the fusion of accumulating vesicles with the end bulb membrane.
-
The intrinsic difference in growth cone regenerative capability that is observed between mammalian CNS and PNS axons may be due to differences in the extent of depolymerization of the cytoskeleton after axotomy, signalling pathways, transport of growth-related molecules and/or the propensity for local protein translation.
Abstract
The assembly of a new growth cone is a prerequisite for axon regeneration after injury. Creation of a new growth cone involves multiple processes, including calcium signalling, restructuring of the cytoskeleton, transport of materials, local translation of messenger RNAs and the insertion of new membrane and cell surface molecules. In axons that have an intrinsic ability to regenerate, these processes are executed in a timely fashion. However, in axons that lack regenerative capacity, such as those of the mammalian CNS, several of the steps that are required for regeneration fail, and these axons do not begin the growth process. Identification of the points of failure can suggest targets for promoting regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schwab, M. E. Functions of Nogo proteins and their receptors in the nervous system. Nature Rev. Neurosci. 11, 799–811 (2010).
Schwab, M. E. How hard is the CNS hardware? Nature Neurosci. 13, 1444–1446 (2010).
Fitch, M. T. & Silver, J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 (2008).
Kwok, J. C., Afshari, F., Garcia-Alias, G. & Fawcett, J. W. Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC. Restor. Neurol. Neurosci. 26, 131–145 (2008).
Pasterkamp, R. J. & Verhaagen, J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Phil. Trans. R. Soc. B 361, 1499–1511 (2006).
Park, K. K., Liu, K., Hu, Y., Kanter, J. L. & He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 223, 45–50 (2010).
Hannila, S. S. & Filbin, M. T. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 209, 321–332 (2008).
Wang, Z. & Jin, Y. Genetic dissection of axon regeneration. Curr. Opin. Neurobiol. 21, 189–196 (2010).
Tom, V. J., Steinmetz, M. P., Miller, J. H., Doller, C. M. & Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539 (2004).
Erturk, A., Hellal, F., Enes, J. & Bradke, F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J. Neurosci. 27, 9169–9180 (2007). This live imaging study pinpoints microtubule disassembly as a key intracellular event in injured axons that prevents axonal regeneration after spinal cord injury.
Wanner, M. et al. Reevaluation of the growth-permissive substrate properties of goldfish optic nerve myelin and myelin proteins. J. Neurosci. 15, 7500–7508 (1995).
Gaze, R. M. The Formation of Nerve Connections (Academic, London, New York, 1970).
Lurie, D. I. & Selzer, M. E. Axonal regeneration in the adult lamprey spinal cord. J. Comp. Neurol. 306, 409–416 (1991).
Wu, Z. et al. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc. Natl Acad. Sci. USA 104, 15132–15137 (2007).
Friede, R. L. & Bischhausen, R. The fine structure of stumps of transected nerve fibers in subserial sections. J. Neurol. Sci. 44, 181–203 (1980).
Morris, J. H., Hudson, A. R. & Weddell, G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the “regenerating unit”. Z. Zellforsch. Mikrosk. Anat. 124, 103–130 (1972).
Pan, Y. A., Misgeld, T., Lichtman, J. W. & Sanes, J. R. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J. Neurosci. 23, 11479–11488 (2003).
Windle, W. F. Inhibition of regeneration of severed axons in the spinal cord. Exp. Neurol. 69, 209–211 (1980).
Kerschensteiner, M., Schwab, M. E., Lichtman, J. W. & Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 11, 572–577 (2005).
Ylera, B. et al. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr. Biol. 19, 930–936 (2009). The authors cut single axons (using a two-photon laser) in the spinal cords of living mice and studied growth cone formation and subsequent axon growth.
Richardson, P. M. & Issa, V. M. Peripheral injury enhances central regeneration of primary sensory neurones. Nature 309, 791–793 (1984).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Knoferle, J. et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl Acad. Sci. USA 107, 6064–6069 (2010).
Goslin, K. & Banker, G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell. Biol. 108, 1507–1516 (1989).
Dotti, C. G. & Banker, G. A. Experimentally induced alteration in the polarity of developing neurons. Nature 330, 254–256 (1987).
Chuckowree, J. A. & Vickers, J. C. Cytoskeletal and morphological alterations underlying axonal sprouting after localized transection of cortical neuron axons in vitro. J. Neurosci. 23, 3715–3725 (2003).
Bradke, F. & Dotti, C. G. Differentiated neurons retain the capacity to generate axons from dendrites. Curr. Biol. 10, 1467–1470 (2000).
Goslin, K. & Banker, G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 108, 1507–1516 (1989).
Bradke, F. & Dotti, C. G. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 10, 574–581 (2000).
Gomis-Ruth, S., Wierenga, C. J. & Bradke, F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr. Biol. 18, 992–1000 (2008).
Verma, P. et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J. Neurosci. 25, 331–342 (2005). Study showing that efficient regeneration of sensory axon growth cones requires local protein translation and degradation, but that adult CNS axons lack ribosomes.
Chierzi, S., Ratto, G. M., Verma, P. & Fawcett, J. W. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur. J. Neurosci. 21, 2051–2062 (2005).
Goldberg, J. L. et al. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 33, 689–702 (2002).
Forscher, P., Lin, C. H. & Thompson, C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature 357, 515–518 (1992).
Spira, M. E., Oren, R., Dormann, A. & Gitler, D. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J. Comp. Neurol. 457, 293–312 (2003).
Sahly, I., Khoutorsky, A., Erez, H., Prager-Khoutorsky, M. & Spira, M. E. On-line confocal imaging of the events leading to structural dedifferentiation of an axonal segment into a growth cone after axotomy. J. Comp. Neurol. 494, 705–720 (2006).
Erez, H. et al. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J. Cell Biol. 176, 497–507 (2007). Live confocal microscope imaging documentation of the sequence of events leading to the polar reorientation of microtubules after axotomy and the ensuing accumulation of Golgi-derived vesicles and endocytic vesicles in separate traps.
Nix, P., Hisamoto, N., Matsumoto, K. & Bastiani, M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc. Natl Acad. Sci. USA 108, 10738–10743 (2011).
Kulbatski, I., Cook, D. J. & Tator, C. H. Calcium entry through L-type calcium channels is essential for neurite regeneration in cultured sympathetic neurons. J. Neurotrauma 21, 357–374 (2004).
Kamber, D., Erez, H. & Spira, M. E. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp. Neurol. 219, 112–125 (2009). Live confocal imaging of the subcellular events underlying end bulb formation and its rescue by calpain cleavage of the submembrane spectrin skeleton.
Ghosh-Roy, A., Wu, Z., Goncharov, A., Jin, Y. & Chisholm, A. D. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J. Neurosci. 30, 3175–3183 (2010).
Ziv, N. E. & Spira, M. E. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J. Neurophysiol. 74, 2625–2637 (1995).
Ziv, N. E. & Spira, M. E. Localized and transient elevations of intracellular Ca2+ induce the dedifferentiation of axonal segments into growth cones. J. Neurosci. 17, 3568–3579 (1997).
Ambron, R. T. & Walters, E. T. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Mol. Neurobiol. 13, 61–79 (1996).
Vogelaar, C. F. et al. Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones. Mol. Cell. Neurosci. 42, 102–115 (2009).
Yan, D., Wu, Z., Chisholm, A. D. & Jin, Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 138, 1005–1018 (2009). Real-time imaging study of axon regeneration in C. elegans implicating local translation and signalling in successful axon regeneration.
Spira, M. E., Benbassat, D. & Dormann, A. Resealing of the proximal and distal cut ends of transected axons: electrophysiological and ultrastructural analysis. J. Neurobiol. 24, 300–316 (1993).
Ashery, U., Penner, R. & Spira, M. E. Acceleration of membrane recycling by axotomy of cultured Aplysia neurons. Neuron 16, 641–651 (1996).
Ziv, N. E. & Spira, M. E. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. Eur. J. Neurosci. 5, 657–668 (1993).
Malkinson, G. & Spira, M. E. Imaging and analysis of evoked excitatory-postsynaptic-calcium-transients by individual presynaptic-boutons of cultured Aplysia sensorimotor synapse. Cell Calcium 47, 315–325 (2010).
Mandolesi, G., Madeddu, F., Bozzi, Y., Maffei, L. & Ratto, G. M. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 18, 1934–1936 (2004). Description of the changes in calcium and electrical activity in mammalian CNS axons after axotomy.
Friel, D. D. & Chiel, H. J. Calcium dynamics: analyzing the Ca2+ regulatory network in intact cells. Trends Neurosci. 31, 8–19 (2008).
Navarro, X., Vivo, M. & Valero-Cabre, A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 82, 163–201 (2007).
Rishal, I. & Fainzilber, M. Retrograde signaling in axonal regeneration. Exp. Neurol. 223, 5–10 (2010).
Enes, J. et al. Electrical activity suppresses axon growth through Cav1.2 channels in adult primary sensory neurons. Curr. Biol. 20, 1154–1164 (2010).
Fishman, H. M. & Bittner, G. D. Vesicle-mediated restoration of a plasmalemmal barrier in severed axons. News Physiol. Sci. 18, 115–118 (2003).
McNeil, P. L. & Kirchhausen, T. An emergency response team for membrane repair. Nature Rev. Mol. Cell Biol. 6, 499–505 (2005).
Yawo, H. & Kuno, M. How a nerve fiber repairs its cut end: involvement of phospholipase A2. Science 222, 1351–1353 (1983).
Bittner, G. D. & Fishman, H. M. in Axonal Regeneration in the Central Nervous System (eds Ingoglia, N. A. & Murray, M.) 337–370 (Marcel Dekker, New York, 2000).
Krause, T. L., Fishman, H. M., Ballinger, M. L. & Bittner, G. D. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J. Neurosci. 14, 6638–6651 (1994).
Regehr, W. G. & Tank, D. W. Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J. Neurosci. 12, 4202–4223 (1992).
Xie, X. Y. & Barrett, J. N. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca2+-triggered protease activity and cytoskeletal disassembly. J. Neurosci. 11, 3257–3267 (1991).
Yoo, S., Nguyen, M. P., Fukuda, M., Bittner, G. D. & Fishman, H. M. Plasmalemmal sealing of transected mammalian neurites is a gradual process mediated by Ca2+-regulated proteins. J. Neurosci. Res. 74, 541–551 (2003).
Benbassat, D. & Spira, M. E. Survival of isolated axonal segments in culture: morphological, ultrastructural, and physiological analysis. Exp. Neurol. 122, 295–310 (1993).
Erez, H. & Spira, M. E. Local self-assembly mechanisms underlie the differential transformation of the proximal and distal cut axonal ends into functional and aberrant growth cones. J. Comp. Neurol. 507, 1019–1030 (2008). An analysis of the structural reorganization of the distal segment of a cut axon and the mechanisms underlying its failure to reassemble a competent growth cone.
George, E. B., Glass, J. D. & Griffin, J. W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 15, 6445–6452 (1995).
Gitler, D. & Spira, M. E. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 20, 1123–1135 (1998).
Gitler, D. & Spira, M. E. Short window of opportunity for calpain induced growth cone formation after axotomy of Aplysia neurons. J. Neurobiol. 52, 267–279 (2002).
Hammarlund, M., Nix, P., Hauth, L., Jorgensen, E. M. & Bastiani, M. Axon regeneration requires a conserved MAP kinase pathway. Science 323, 802–806 (2009). A study showing that growth cone regeneration in C. elegans requires an MAPK signalling pathway.
Liu, H. et al. Matrix metalloproteinase inhibition enhances the rate of nerve regeneration in vivo by promoting dedifferentiation and mitosis of supporting schwann cells. J. Neuropathol. Exp. Neurol. 69, 386–395 (2010).
Park, B. et al. Impairment of protein trafficking upon overexpression and mutation of optineurin. PLoS ONE 5, e11547 (2010).
Sivasankaran, R. et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nature Neurosci. 7, 261–268 (2004).
Prager-Khoutorsky, M. & Spira, M. E. Neurite retraction and regrowth regulated by membrane retrieval, membrane supply, and actin dynamics. Brain Res. 1251, 65–79 (2009).
Schaefer, A. W. et al. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev. Cell 15, 146–162 (2008).
Shaw, G. & Bray, D. Movement and extension of isolated growth cones. Exp. Cell Res. 104, 55–62 (1977).
Baas, P. W. & Heidemann, S. R. Microtubule reassembly from nucleating fragments during the regrowth of amputated neurites. J. Cell Biol. 103, 917–927 (1986).
Sheetz, M. P., Sable, J. E. & Dobereiner, H. G. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 35, 417–434 (2006).
Carter, J. M., Demizieux, L., Campenot, R. B., Vance, D. E. & Vance, J. E. Phosphatidylcholine biosynthesis via CTP:phosphocholine cytidylyltransferase 2 facilitates neurite outgrowth and branching. J. Biol. Chem. 283, 202–212 (2008).
Vance, J. E., Karten, B. & Hayashi, H. Lipid dynamics in neurons. Biochem. Soc. Trans. 34, 399–403 (2006).
Sotelo-Silveira, J. R., Calliari, A., Kun, A., Koenig, E. & Sotelo, J. R. RNA trafficking in axons. Traffic 7, 508–515 (2006).
Yoo, S., van Niekerk, E. A., Merianda, T. T. & Twiss, J. L. Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp. Neurol. 223, 19–27 (2010).
Leung, K. M. et al. Asymmetrical β-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nature Neurosci. 9, 1247–1256 (2006).
Gumy, L. F. et al. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17, 85–98 (2010).
Taylor, A. M. et al. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J. Neurosci. 29, 4697–4707 (2009).
Court, F. A., Hendriks, W. T., MacGillavry, H. D., Alvarez, J. & van Minnen, J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 28, 11024–11029 (2008).
Hellal, F. et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931 (2011). This study shows that administration of the clinically approved drug taxol to rats with spinal cord injuries causes axon regeneration and improvement in gait by reducing scarring and inducing axon regeneration.
Bradke, F. & Dotti, C. G. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19, 1175–1186 (1997).
Zakharenko, S. & Popov, S. Dynamics of axonal microtubules regulate the topology of new membrane insertion into the growing neurites. J. Cell Biol. 143, 1077–1086 (1998).
Futerman, A. H. & Banker, G. A. The economics of neurite outgrowth — the addition of new membrane to growing axons. Trends Neurosci. 19, 144–149 (1996).
Richardson, P. M. & Verge, V. M. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 411, 406–408 (1987).
Shoemaker, S. E., Sachs, H. H., Vaccariello, S. A. & Zigmond, R. E. A conditioning lesion enhances sympathetic neurite outgrowth. Exp. Neurol. 194, 432–443 (2005).
Cafferty, W. B. et al. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J. Neurosci. 24, 4432–4443 (2004).
Chong, M. S. et al. The downregulation of GAP-43 is not responsible for the failure of regeneration in freeze-killed nerve grafts in the rat. Exp. Neurol. 129, 311–320 (1994).
Mason, M. R., Lieberman, A. R., Grenningloh, G. & Anderson, P. N. Transcriptional upregulation of SCG10 and CAP-23 is correlated with regeneration of the axons of peripheral and central neurons in vivo. Mol. Cell. Neurosci. 20, 595–615 (2002).
Nilsson, A., Moller, K., Dahlin, L., Lundborg, G. & Kanje, M. Early changes in gene expression in the dorsal root ganglia after transection of the sciatic nerve; effects of amphiregulin and PAI-1 on regeneration. Mol. Brain Res. 136, 65–74 (2005).
Rasband, M. N. The axon initial segment and the maintenance of neuronal polarity. Nature Rev. Neurosci. 11, 552–562 (2010).
Nishimura, K., Akiyama, H., Komada, M. & Kamiguchi, H. βIV-spectrin forms a diffusion barrier against L1CAM at the axon initial segment. Mol. Cell. Neurosci. 34, 422–430 (2007).
Nishimura, T. & Goll, D. E. Binding of calpain fragments to calpastatin. J. Biol. Chem. 266, 11842–11850 (1991).
Hirokawa, N., Noda, Y., Tanaka, Y. & Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nature Rev. Mol. Cell Biol. 10, 682–696 (2009).
Hammond, J. W. et al. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572–583 (2010).
Kapitein, L. C. et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20, 290–299 (2010).
Schafer, D. P. et al. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 29, 13242–13254 (2009).
Konishi, Y. & Setou, M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nature Neurosci. 12, 559–567 (2009).
Sengottuvel, V., Leibinger, M., Pfreimer, M., Andreadaki, A. & Fischer, D. Taxol facilitates axon regeneration in the mature CNS. J. Neurosci. 31, 2688–2699 (2011).
Acknowledgements
This Review was written as a result of a meeting sponsored by the UK Academic Study Group and the Institute of Advanced Studies of the Hebrew University of Jerusalem, Israel. The laboratory of M.E.S is supported by grants from The Israel Science Foundation, The Israel Ministry of Health, The United States–Israel Binational Science Foundation, the European Commission and the Charles E. Smith Family and Professor Joel Elkes Laboratory for Collaborative Research in Psychobiology. The laboratory of F.B. is supported by the Deutsches Zentrum für Neurodegenerative Erkrankungen, the Deutsche Forschungsgemeinschaft, the International Foundation for Research in Paraplegia and the Human Frontier Science Program. J.W.F. is supported by the UK Medical Research Council, Engineering and Physical Sciences Research Council, European Union Framework 7 Programme Spinal Cord Repair, Plasticise and Angioscaff, Henry Smith Charity, Christopher and Dana Reeve Foundation, and the UK National Institute of Health Research Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
James Fawcett is a paid consultant for Acorda Therapeutics, Novartis and Covidien. Frank Bradke and Micha E. Spira declare no competing financial interests.
Supplementary information
Supplementary Information Figure S1
Formation of vesicles traps by microtubules restructuring after axotomy. (PDF 5338 kb)
Supplementary Information S2 (movie)
Uniform microtubule plus end orientation in a control axon of a cultured Aplysia californica neuron. (AVI 1923 kb)
Supplementary Information S3 (movie)
The formation of microtubule-based vesicle traps after axotomy. (AVI 5107 kb)
Supplementary Information S4 (movie)
Accumulation of organelles in the microtubule-based vesicle traps after axotomy. (AVI 4660 kb)
Supplementary Information S5 (movie)
The spatiotemporal relationships between the formation of microtubule-based vesicle traps and the accumulation of organelles. (AVI 7908 kb)
Related links
Glossary
- Local protein translation
-
Mammalian peripheral nervous system axons and axons in many invertebrate species contain ribosomes, messenger RNAs and a Golgi apparatus or equivalent. In such axons, proteins can be synthesized in the axon tip, and if local translation is prevented, the regeneration of a cut axon is inhibited.
- Taxol
-
Taxol is a compound that at low concentrations promotes the polymerization of tubulin into microtubules and stabilizes microtubules against depolymerization, and hence may promote axon growth over inhibitory substrates.
- The conditioning response
-
A severed peripheral nervous system axon will begin to regenerate after a few hours. If the same axon is cut again 2 or more days later, the speed of axon regeneration increases. This phenomenon is known as the conditioning response.
- Axon initial segment
-
The axon initial segment is the part of the axon that is closest to the cell body and is the point of initiation for action potentials. It may also act as a selective transport filter for some types of axonal cargo.
Rights and permissions
About this article
Cite this article
Bradke, F., Fawcett, J. & Spira, M. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13, 183–193 (2012). https://doi.org/10.1038/nrn3176
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3176
This article is cited by
-
Unleashing Axonal Regeneration Capacities: Neuronal and Non-neuronal Changes After Injuries to Dorsal Root Ganglion Neuron Central and Peripheral Axonal Branches
Molecular Neurobiology (2024)
-
Discovery of therapeutic targets for spinal cord injury based on molecular mechanisms of axon regeneration after conditioning lesion
Journal of Translational Medicine (2023)
-
Neural stem/progenitor cells from adult canine cervical spinal cord have the potential to differentiate into neural lineage cells
BMC Veterinary Research (2023)
-
Microfluidic devices as model platforms of CNS injury-ischemia to study axonal regeneration by regulating mitochondrial transport and bioenergetic metabolism
Cell Regeneration (2022)
-
Mitochondrial function in spinal cord injury and regeneration
Cellular and Molecular Life Sciences (2022)