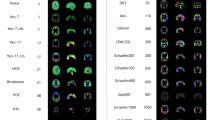
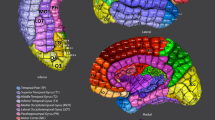
Key Points
-
Brain atlases are reference systems that associate neuroanatomical labels (nomenclature) with canonical representations of anatomy in a three-dimensional coordinate system. These systems commonly integrate multisubject data from many different sources (for example, histology, functional MRI and positron emission tomography), and could provide statistical representations of anatomy and function in whole populations.
-
Initially, brain atlases were purely neuroanatomical, based on a single, often sparsely sampled, representative example. Now, brain atlases include population statistics on structure, gene expression, receptor patterns or connectivity over time.
-
Modern cytoarchitectonic studies are using computational methods to pool information across subjects on such features as receptor distributions, myelination characteristics and cellular content. Correlations between functional activation and the underlying cyto- or myeloarchitecture can then be tested by using these architectural maps to define regions of interest in functional imaging studies.
-
Integration of cytoarchitectural maps from many subjects has allowed classical maps to be re-evaluated and corrected. It has also led to a quantitative description of the intersubject variability of cytoarchitectonic areas and to the discovery of hitherto unknown cytoarchitectonic areas in the intraparietal, secondary somatosensory and extrastriate cortex.
-
Diffusion tensor imaging (DTI) provides a new source of image contrast to map white matter integrity and connectivity, and has opened up new opportunities for brain mapping and atlasing.
-
Population-based atlases can average anatomical features across individuals, revealing generic features that are not identifiable in individual representations owing to their considerable variability. They have identified group-specific patterns of brain structure in Alzheimer's disease, HIV/AIDS, schizophrenia, in methamphetamine users, and in developmental disorders such as fetal alcohol syndrome and Williams syndrome.
-
Brain atlases are beginning to be used in clinical studies, including drug trials of antipsychotics or mood stabilizers, to investigate factors that influence disease expression and therapeutic response.
-
In the next decade, population-based atlases will probably gain widespread applicability in genetic studies. Data from twins and those at genetic risk for specific diseases have been incorporated into brain atlasing studies to discover previously unknown effects on the brain of variations at specific genetic loci.
Abstract
Atlases of the human brain have an important impact on neuroscience. The emergence of ever more sophisticated imaging techniques, brain mapping methods and analytical strategies has the potential to revolutionize the concept of the brain atlas. Atlases can now combine data describing multiple aspects of brain structure or function at different scales from different subjects, yielding a truly integrative and comprehensive description of this organ. These integrative approaches have provided significant impetus for the human brain mapping initiatives, and have important applications in health and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Toga, A. W. & Mazziotta, J. C. in Brain Mapping: the Methods (eds Toga, A. W. & Mazziotta, J.C.) 3–25 (Academic, San Diego, 1996).
Duvernoy, H. M. The Human Brain (Springer, New York, 1991).
Ono, M., Kubik, S. & Abernathey, C. D. Atlas of the Cerebral Sulci (Thieme, Stuttgart, 1990). This widely used atlas reports anatomical descriptions and trends in individual variabilities of cortical sulci. It serves as a reminder of the challenges in developing multisubject reference systems for human brain mapping.
Talairach, J. & Szikla, G. Atlas d'Anatomie Stereotaxique du Telencephale: Etudes Anatomo-Radiologiques (Masson & Cie, Paris, 1967) (in French).
Talairach, J. & Tournoux, P. Co-planar Stereotaxic Atlas of the Human Brain (Thieme, New York, 1988). This atlas influenced the brain mapping field by providing a principled method for spatially transforming anatomical datasets into a coordinate-based reference system based on the anterior commissure–posterior commisure line. The atlas presents post-mortem data from a single subject.
Brodmann, K. in Some Papers on the Cerebral Cortex, translated as: On the Comparative Localization of the Cortex 201–230 (Thomas, Springfield, Illinois, 1960).
von Economo, C. & Koskinas, G. N. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen (Springer, Berlin, 1925) (in German).
Flechsig, P. Anatomie des menschlichen Gehirns und Rückenmarks auf myelogenetischer Grundlage (Thieme, Leipzig, 1920) (in German).
Smith, G. E. A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relation-ship to the cerebral sulci. J. Anat. 41, 237–254 (1907).
Vogt, C. & Vogt, O. Allgemeinere ergebnisse unserer hirnforschung. J. Psychol. Neurol. 25, 292–398 (1919) (in German).
Mai, J., Assheuer, J. & Paxinos, G. Atlas of the Human Brain (Academic, San Diego, 1997).
Matsui, T. & Hirano, A. An Atlas of the Human Brain for Computerized Tomography (Igako-Shoin,Tokyo, 1978).
Schaltenbrand, G. & Bailey, P. Introduction to Stereotaxis with an Atlas of the Human Brain (Thieme, Stuttgart & New York, 1959).
Schaltenbrand, G. & Wahren, W. Atlas for Stereotaxy of the Human Brain 2nd edn (Thieme, Stuttgart, 1977).
Van Buren, J. M. & Borke, R. C. Variations and Connections of the Human Thalamus Vols 1 & 2 (Springer, New York, 1972).
Van Buren, J. M. & Maccubin, D. An outline atlas of human basal ganglia and estimation of anatomic variants. J. Neurosurg. 19, 811–839 (1962).
Mansour, A., Fox, C. A., Burke, S., Akil, H. & Watson, S. J. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J. Chem. Neuroanat. 8, 283–305 (1995).
Dejerine, J. Anatomie des Centres Nerveux (Rueff, Paris, 1901).
Damasio, H. Human Brain Anatomy in Computerized Images (Oxford Univ. Press, Oxford & New York, 1995).
Mori, S., Wakana, S., Nagae-Poetscher, L. M. & van Zijl, P. C. MRI Atlas of Human White Matter (Elsevier Science, Amsterdam, 2005).
Toga, A. W. Brain Warping (Academic, San Diego, 1998). The author surveys the many approaches and computational algorithms for deforming brain imaging data from multiple subjects or modalities to match an atlas or other standardized coordinate space.
Zilles, K. et al. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Europ. Neuropsychopharmacol. 12, 587–599 (2002).
Brodmann, K. Physiologie des Gehirns. Neue Dtsch Chir. 11, 85–426 (1914) (in German).
Bailey, P. & von Bonin, G. The Isocortex of Man (Univ. Illinois Press, Urbana, 1951).
Sanides, F. Die Architektonik des menschlichen Stirnhirns (Springer, Berlin & New York, 1962)(in German).
Sarkisov, S. A., Filimonoff, I. N., Kononowa, E. P., Preobrachenskaja, I. S. & Kukuew, L. A. Atlas of the Cytoarchitectonics of the Human Cerebral Cortex (Medgiz, Moscow, 1955).
Zilles, K., Armstrong, E., Schleicher, A. & Kretschmann, H. J. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. 179, 173–179 (1988).
Nelissen, K., Luppino, G., Vanduffel, W., Rizzolatti, G. & Orban, G. A. Observing others: multiple action representation in the frontal lobe. Science 310, 332–336 (2005).
Sereno, M. I. et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893 (1995).
Bremmer, F. et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29, 287–296 (2001).
Zeki, S. et al. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 11, 641–649 (1991).
Lashley, K. S. & Clark, G. The cytoarchitecture of the cerebral cortex of atlases: a critical examination of architectonic studies. J. Comp. Neurol. 85, 223–306 (1946).
Luppino, G. & Rizzolatti, G. The organization of the frontal motor cortex. News Physio. Sci. 15, 219–224 (2000).
Zilles, K., Schleicher, A., Palomero-Gallagher, N. & Amunts, K. in Brain Mapping: The Methods 2nd edn (eds Toga, A. W. & Mazziotta, J. C.) 573–602 (Academic, Amsterdam,2002).
Amunts, K. et al. Broca's region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 412, 319–341 (1999).
Amunts, K., Malikovic, A., Mohlberg, H., Schormann, T. & Zilles, K. Brodmann's areas 17 and 18 brought into stereotaxic space — where and how variable? NeuroImage 11, 66–84 (2000).
Zilles, K. et al. Quantitative analysis of sulci in the human cerebral cortex: development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum. Brain Mapp. 5, 218–221 (1997).
Tootell, R. B. H. et al. Functional analysis of primary visual cortex (V1) in humans. Proc. Natl Acad. Sci. USA 95, 811–817 (1998).
Talavage T. M. et al. Tonotopic organization in human auditory cortex revealed by progressions of frequency sensitivity. J. Neurophysiol. 91, 1282–1296 (2004).
Geyer, S. et al. Two different areas within the primary motor cortex of man. Nature 382, 805–807 (1996).
Young, J. P. et al. Regional cerebral blood flow correlations of somatosensory areas 3a, 3b, 1, and 2 in humans during rest: a PET and cytoarchitectural study. Hum. Brain Mapp. 19, 183–196 (2003).
Hagler, D. & Sereno, M. I. Spatial maps in frontal and prefrontal cortex. Neuroimage 29, 567–577 (2005).
Van Essen, D. C. Surface-based approaches to spatial localization and registration in primate cerebral cortex. NeuroImage 23, S97–S107 (2004). This review article describes many of the neuroanatomical, technical and informatics issues involved in integrating cortically-derived neuroimaging data across subjects and modalities.
Tootell, H. et al. Functional analysis of primary visual cortex (V1) in humans. Proc. Natl Acad. Sci. 95, 811–817 (1998).
Larsson, J. et al. Neuronal correlates of real and illusory contour perception: functional anatomy with PET. Europ. J. Neurosci. 11, 4024–4036 (1999).
Schleicher, A., Amunts, K., Geyer, S., Morosan, P. & Zilles, K. Observer-independent method for microstructural parcellation of cerebral cortex: a quantitative approach to cytoarchitectonics. Neuroimage 9, 165–177 (1999).
Schleicher, A. et al. A stereological approach to human cortical architecture: identification and delineation of cortical areas. J. Chem. Neuroanat. 20, 31–47 (2000).
Schleicher, A. et al. Quantitative architectonic analysis: a new approach to cortical mapping. Anat. Embryol. 210, 373–386 (2005).
Annese, J. & Toga, A. W. in Brain Mapping: The Methods (eds Toga, A. W. & Mazziotta, J. C.) 537–564 (Academic, San Diego, 2002).
Hömke, L. in Numerical Linear Algebra with Applications 215–229 (Wiley, Copper Mountain, 2006).
Thompson, P. & Toga, A. W. in Handbook of Medical Image Processing (ed. Bankman, I.) 159–170 (Academic, San Diego, 2000).
Roland P. E. & Zilles K. Brain atlases — a new research tool. Trends Neurosci. 17, 458–467 (1994).
Mazziotta, J. C. et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philos. Trans. R. Soc. Lond., B, Biol. Sci. 356, 1293–1322 (2001). A description of an international consortium project that was set up to develop a probabilistic reference system for the human brain, incorporating statistical information on the variations in human brain structure and function in a population of 7000 subjects.
Evans, A. C., Collins, D. L. & Milner, B. An MRI-based stereotactic brain atlas from 300 young normal subjects. Soc. Neurosci. Abstr. 408 (1992).
Eickhoff, S. et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25, 1325–1335 (2005).
Grefkes, C., Geyer, S., Schormann, T., Roland, P. & Zilles, K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. NeuroImage 14, 617–631 (2001).
Eickhoff, S., Schleicher, A., Zilles, K. & Amunts, K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb. Cortex 16, 254–267 (2005).
Geyer, S., Schleicher, A. & Zilles, K. Areas 3a, 3b, and 1 of human primary somatosensory cortex: 1. Microstructural organization and interindividual variability. NeuroImage 10, 63–83 (1999).
Choi, H.-J. et al. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J. Comp. Neurol. 495, 53–69 (2006).
Eickhoff, S. B., Weiss, P. H., Amunts, K., Fink, G. R. & Zilles, K. Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum. Brain Mapp. 27, 611–621 (2006).
Malikovic, A. et al. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb. Cortex 7 April 2006 (doi:10.1093/cercor/bhj181).
Amunts, K. et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 210, 342–352 (2005).
Geyer, S., Schormann, T., Mohlberg, H. & Zilles, K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. 2. Spatial normalization to standard anatomical space. NeuroImage 11, 684–696 (2000).
Morosan, P. et al. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage 13, 684–701 (2001).
Rademacher, J. et al. Probabilistic mapping and volume measurement of human primary auditory cortex. NeuroImage 13, 669–683 (2001).
Rademacher, J. Bü rgel, U. & Zilles, K. Stereotaxic localization, intersubject variability and interhemispheric differences of the human auditory thalamocortical system. NeuroImage 17, 142–160 (2002).
Roland, P. E. & Zilles, K. The developing european computerized human brain database for all imaging modalities. NeuroImage 4, 39–47 (1996).
Roland, P. E. et al. Cytoarchitectural maps of the human brain in standard anatomical space. Hum. Brain Mapp. 5, 222–227 (1997).
Zilles, K. & Palomero-Gallagher, N. Cyto-, myelo- and receptor architectonics of the human parietal cortex. NeuroImage 14, 8–20 (2001).
Zilles, K. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J. Anatomy 187, 515–537 (1995).
Amunts, K. et al. Analysis of the neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space — the roles of Brodmann areas 44 and 45 NeuroImage 22, 42–56 (2004).
Bodegård, A. et al. Object shape differences reflected by somatosensory cortical activation. J. Neurosci. 20, 1–5 (2000).
Bodegård, A. et al. Somatosensory areas in man activated by moving stimuli. Cytoarchitectonic mapping and PET. NeuroReport 11, 187–191 (2000).
Eickhoff, S., Amunts, K., Mohlberg, H. & Zilles, K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb. Cortex 16, 268–279 (2006).
Horwitz, B. et al. Activation of Broca's area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia 41, 1868–1876 (2003).
Pazos, A., Probst, A. & Palacios, J. M. Serotonin receptors in the human brain — III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21, 97–122 (1987).
Pazos, A., Probst, A. & Palacios, J. M. Serotonin receptors in the human brain — IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience 21, 123–139 (1987).
Vogt, B. A., Plager, M. D., Crino, P. B. & Bird, E. D. Laminar distributions of muscarinic acetylcholine, serotonin, GABA and opioid receptors in human posterior cingulate cortex. Neuroscience 36, 165–174 (1990).
Zilles, K. in From Monkey Brain to Human Brain (eds Dehaene, S., Duhamel, J.-R., Hauser, M. & Rizzolatti, G.) 41–56 (MIT Press, Cambridge, Massachusetts, 2005).
Zilles, K., Palomero-Gallagher, N. & Schleicher, A. Transmitter receptors and functional anatomy of the cerebral cortex. J. Anat. 205, 417–432 (2004).
Geyer, S., Schleicher, A. & Zilles, K. The somatosensory cortex of human: cytocarchitecture and regional distributions of receptor-binding sites. NeuroImage 6, 27–45 (1997).
Morosan, P., Rademacher, J., Palomero-Gallagher, N. & Zilles, K. in The Auditory Cortex: Towards a Synthesis of Human and Animal Research (eds König, R., Heil, P., Budinger, E. & Scheich, H.) 27–50 (Lawrence Erlbaum, Mahwah, New Jersey, 2005).
Basser, P. J., Mattiello, J. & LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 103, 247–254 (1994).
Conturo, T. E., McKinstry, R. C., Akbudak, E. & Robinson, B. H. Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magn. Reson. Med. 35, 399–412 (1996).
Pierpaoli, C. & Basser, P. J. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 36, 893–906 (1996).
Ulug, A. M., Bakht, O., Bryan R. N. & van Zijl, P. C. M. Mapping of Human Brain Fibers Using Diffusion Tensor Imaging. Proc. Int. Soc. Mag. Reson. Med. 4, 1325 (1996)
Douek, P., Turner, R., Pekar, J., Patronas, N. & Le Bihan, D. MR color mapping of myelin fiber orientation. J. Comput. Assist. Tomogr. 15, 923–929 (1991).
Hsu, E. W. & Mori, S. Analytical interpretations of NMR diffusion measurements in an anisotropic medium and a simplified method for determining fiber orientation. Magn. Reson. Med. 34, 194–200 (1995).
Nakada, T. & Matsuzawa, H. Three-dimensional anisotropy contrast magnetic resonance imaging of the rat nervous system: MR axonography. Neurosci. Res. 22, 389–398 (1995).
Makris, N. et al. Morphometry of in vivo human white matter association pathways with diffusion weighted magnetic resonance imaging. Ann. Neurol. 42, 951–962 (1997). A monumental work in which DTI-based image contrast was correlated with white matter anatomy in a comprehensive manner for the first time.
Pajevic, S. & Pierpaoli, C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn. Reson. Med. 42, 526–540 (1999).
Conturo, T. E. et al. Tracking neuronal fiber pathways in the living human brain. Proc. Natl Acad. Sci. USA 96, 10422–10427 (1999).
Jones, D. K., Simmons, A., Williams S. C. & Horsfield, M. A. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn. Reson. Med. 42, 37–41 (1999).
Mori, S., Crain, B. J., Chacko, V. P. & van Zijl, P. C. M. Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annal. Neurol. 45, 265–269 (1999).
Xue, R., van Zijl, P. C. M, Crain, B. J., Solaiyappan, M. & Mori, S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn. Reson. Med. 42, 1123–1127 (1999).
Basser, P. J., Pajevic, S., Pierpaoli, C., Duda, J. & Aldroubi, A. In vitro fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632 (2000).
Poupon, C. et al. Regularization of diffusion-based direction maps for the tracking of brain white matter fascicules. NeuroImage 12, 184–195 (2000).
Lazar, M. et al. White matter tractography using diffusion tensor deflection. Hum. Brain Mapp. 18, 306–321 (2003).
Behrens, T. E. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neurosci. 6, 750–757 (2003).
Ludwig, E. & Klingler, J. Atlas Cerebrei Human: the Internal Structure of the Brain Demonstrated on the Basis of Macroscopical Preparations (Karger, Basel, 1956).
Stieltjes, B. et al. Diffusion tensor imaging and axonal tracking in the human brainstem. NeuroImage 14, 723–735 (2001).
Catani, M., Howard, R. J., Pajevic, S. & Jones, D. K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage 17, 77–94 (2002).
Mori, S. et al. Imaging cortical association tracts in human brain. Magn. Reson. Med. 47, 215–223 (2002).
Wakana, S., Jiang, H., Nagae-Poetscher, L. M., Van Zijl, P. C. & Mori, S. Fiber tract-based atlas of human white matter anatomy. Radiology 230, 77–87 (2004). A comprehensive atlas of the human white matter based on two-dimensional and three-dimensional visualization of DTI data.
Mamata, H. et al. High-resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. Am. J. Neuroradiol. 23, 67–75 (2002).
Alexander, D. C., Pierpaoli, C., Basser, P. J. & Gee, J. C. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans. Med. Imaging 20, 1131–1139 (2001).
Jones, D. K. et al. Spatial normalization and averaging of diffusion tensor MRI data sets. Neuroimage 17, 592–617 (2002).
Xu, D., Mori, S., Shen, D., van Zijl, P. C. & Davatzikos, C. Spatial normalization of diffusion tensor fields. Magn. Reson. Med. 50, 175–182 (2003).
Pagani, E., Filippi, M., Rocca, M. A. & Horsfield, M. A. A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. NeuroImage 26, 258–265 (2005).
Pierpaoli, C. et al. Water diffusion change in Wallerian degeneration and their dependence on white matter architecture. NeuroImage 13, 1174–1185 (2001).
Thomalla G. et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage 22, 1767–1774 (2004).
Lee, J. S., Han, M. K., Kim, S. H., Kwon, O. K. & Kim, J. H. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: topographical correlation with clinical symptoms. NeuroImage 26, 771–776 (2005).
Johansen-Berg, H. et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc. Natl Acad. Sci. USA 101, 13335–13340 (2004).
Frank, L. R. Anisotropy in high angular resolution diffusion-weighted MRI. Magn. Reson. Med. 45, 935–939 (2001).
Tournier, J. D., Calamante, F., Gadian, D. G. & Connelly, A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage 23, 1176–1185 (2004).
Tuch, D. S., Reese, T. G., Wiegell, M. R. & Wedeen, V. J. Diffusion MRI of complex neural architecture. Neuron 40, 885–895 (2003).
Wedeen, V. J., Hagmann, P., Tseng, W. Y., Reese, T. G. & Weisskoff, R. M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn. Reson. Med. 54, 1377–1386 (2005).
Bürgel, U., Schormann, T., Schleicher, A. & Zilles, K. Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: position and spatial variability of the optic radiation. NeuroImage 10, 489–499 (1999).
Rademacher, J., Engelbrecht, V., Bü rgel, U., Freund, H. J. & Zilles, K. Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. NeuroImage 9, 393–406 (1999).
Rademacher, J. et al. Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain 124, 2232–2258 (2001).
Mega, M. S. et al. Mapping pathology to metabolism: coregistration of stained whole brain sections to PET in alzheimer's disease. NeuroImage 5, 147–153 (1997).
Toga, A. W., Ambach, K. L., Quinn, B. C., Hutchin, M. & Burton, J. S. Postmortem anatomy from cryosectioned whole human brain. J. Neurosci. Methods 5, 239–252 (1994).
Teipel, S. J. et al. Measurement of basal forebrain atrophy in Alzheimer's disease using MRI. Brain 128, 2626–2644 (2005).
Toga, A. W. & Thompson, P. M. Mapping brain asymmetry. Nature Rev. Neurosci. 4, 37–38 (2003).
Toga, A. W. & Thompson, P. M. Genetics of brain structure and intelligence. Ann. Rev. Neurosci. 28, 1–23 (2005).
Grenander, U. & Miller, M. I. Computational Anatomy: an Emerging Discipline. Technical Report, Dept. Mathematics, Brown Univ. (1998). This highly-cited article was influential in stimulating mathematical developments in the field of computational anatomy. A new framework is proposed that represents anatomical variation by defining statistics and probability measures on three-dimensional elastic or fluid mappings that deform a canonical template of anatomy.
Narr, K. L. et al. Mapping morphology of the corpus callosum in schizophrenia. Cereb. Cortex 10, 40–49 (2000).
Thompson, P. M. et al. Cortical variability and asymmetry in normal aging and alzheimer's disease. Cereb. Cortex 8, 492–509 (1998).
Thompson, P. M., Mega, M. S. & Toga, A. W. in Brain Mapping: the Disorders (eds Toga A. W. & Mazziotta, J. C.) 131–177 (Academic, San Diego, 2000).
Sowell, E. R. et al. Mapping cortical change across the human life span. Nature Neurosci. 6, 309–315 (2003).
Levitt, J. G. et al. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol. Psychiatry 54, 1355–1366 (2003).
Thompson, P. M. et al. Cortical complexity and thickness are increased in Williams Syndrome. J. Neurosci. 25, 4146–4158 (2005).
Mazziotta, J. C., Toga, A. W., Evans, A. C., Fox, P. & Lancaster, J. A probabilistic atlas of the human brain: theory and rationale for its development. NeuroImage 2, 89–101 (1995).
Geschwind, N. & Galaburda, A. M. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch. Neurol. 42, 634–656 (1985).
Sowell, E. R. et al. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am. J. Psychiatry, 157, 1475–1484 (2000).
Luders, E. et al. Mapping cortical gray matter in the young adult brain: effects of gender. NeuroImage 26, 493–501 (2005).
Crow, T. J. Handedness, language lateralisation and anatomical asymmetry: relevance of protocadherin XY to hominid speciation and the aetiology of psychosis. Point of view. Br. J. Psychiatry 181, 295–297 (2002).
Narr, K. L. et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb. Cortex 15, 708–719 (2005).
Van Essen, D. C. Surface-based approaches to spatial localization and registration in primate cerebral cortex. NeuroImage 23, S97–S107 (2005).
van Horn, J. D. Neuroimaging databases as a resource for scientific discovery. Int. Rev. Neurobiol. 66, 55–87 (2005).
Giedd, J. N. et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 2, 861–863 (1999).
Rapoport, J. L., Addington, A. & Frangou, S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr. Psychiatry Rep. 7, 81–82 (2005).
Thompson, P. M. et al. Early cortical change in Alzheimer's disease detected with a disease-specific population-based brain atlas. Cereb. Cortex 11, 1–16 (2001).
Cannon, T. D. et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment and their interactions. Schizophr. Bull. 29, 653–669 (2003).
Cannon, T. D. et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter and impaired short- and long-term memory. Arch. Gen. Psychiatry 62, 1205–1213 (2005).
Lin, J. J. 3D Pre-operative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology 65, 1094–1097 (2005).
Moore, G. J., Bebchuk, J. M., Wilds, I. B., Chen, G. & Manji, H. K. Lithium-induced increase in human brain grey matter. Lancet 356, 1241–1242 (2000).
Lieberman, D. Z. & Goodwin, F. K. Use of olanzapine in the treatment of bipolar I disorder. Expert Rev. Neurother. 4, 759–767 (2004).
Leow, A. et al. Brain structural mapping using a novel hybrid implicit/explicit framework based on the level-set method. NeuroImage 24, 910–927 (2005).
Kikinis, R. et al. A digital brain atlas for surgical planning, model-driven segmentation, and teaching. IEEE Trans. Vis. Comput. Graph. 2, 232–241 (1996).
Mangin, J. F. et al. Brain morphometry using 3D moment invariants. Med. Image Anal. 8, 187–196 (2004).
Collins, D. L., Peters, T. M., Evans, A. C. An automated 3D non-linear image deformation procedure for determination of gross morphometric variability in the human brain. Proc. Visualization. Biomed. Comput. 3, 180–190 (1994).
Thompson, P. M. et al. Detection and mapping of abnormal brain structure with a probabilistic atlas of cortical surfaces. J. Comput. Asst. Tomogr. 21, 567–581 (1997).
Chung, M. K. Cortical thickness analysis in autism with heat kernel smoothing. NeuroImage 25, 1256–1265 (2005).
Carmichael, O. T. et al. Mapping ventricular changes related to dementia and mild cognitive impairment in a large community-based cohort. IEEE Int. Symp. Biomed. Imaging 315–318 (2006).
Gogtay, N. et al. Dynamic mapping of human cortical development during childhood and adolescence. Proc. Natl Acad. Sci. 101, 8174–8179 (2004).
Sowell, E. R., Thompson, P. M. & Toga, A. W. Mapping changes in the human cortex throughout the span of life. Neuroscientist 10, 372–392 (2004).
Thompson, P. M. et al. Growth patterns in the developing brain detected by using continuum-mechanical tensor maps. Nature 404, 190–193 (2000).
Lerch, J. P. et al. Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cereb. Cortex 15, 995–1001 (2005).
Janke, A. L. et al. 4D deformation modeling of cortical disease progression in Alzheimer's dementia. Magn. Reson. Med. 46, 661–666 (2001).
Thompson, P. M. et al. Dynamics of gray matter loss in Alzheimer's disease. J. Neurosci. 23, 994–1005 (2003).
Friston, K. J. et al. Spatial registration and normalisation of images. Hum. Brain Mapp. 2, 16 (1995).
van Essen, D. C. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage 28, 635–662 (2005).
Fischl, B. et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage 23, S69–S84 (2004).
Pitiot, A., Delingette, H., Thompson, P. M. & Ayache, N. Expert knowledge-guided segmentation system for brain MRI. NeuroImage 23, S85–S96 (2004).
Zijdenbos, A. P., Lerch, J. P., Bedell, B. J. & Evans, A. C. Brain imaging in drug R&D. Biomarkers 10, S58–S68 (2005). The authors describe a recent extension of the atlas concept to include large-scale processing of images from drug trials, analysing images that have been mapped into a stereotaxic coordinate space.
Duncan, J. S. et al. Geometric strategies for neuroanatomic analysis from MRI. NeuroImage 23, S34–S45 (2004).
Shattuck, D. W., Sandor-Leahy, S. R., Schaper, K. A., Rottenberg, D. A. & Leahy, R. M. Magnetic resonance image tissue classification using a partial volume model. NeuroImage 13, 856–876 (2001).
Dinov, I. D. et al. Analyzing functional brain images in a probabilistic atlas: a validation of sub-volume thresholding. J. Comput. Asst. Tomog. 24, 128–138 (2000).
Rasser, P. E. et al. Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. NeuroImage 26, 941–951 (2005).
Thompson, P. et al. Mapping hippocampal and ventricular change in Alzheimer's disease. NeuroImage 22, 1754–1766 (2004).
Lin, C. L. et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 99, 978–985 (2003).
Ballmaier, M. et al. Localizing gray matter deficits in late onset depression using computational cortical pattern matching methods. Am. J. Psychiatry 161, 2091–2099 (2004).
Cannon, T. D. et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc. Natl Acad. Sci. USA 99, 3228–3233 (2002).
Narr, K. L. et al. Abnormal gyral complexity in first episode schizophrenia. Biol. Psychiatry 55, 859–867 (2004).
Thompson, P. et al. Mapping hippocampal and ventricular change in Alzheimer's disease. NeuroImage 22, 1754–1766 (2004).
Vidal, C. et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with Schizophrenia. Arch. Gen. Psychiatry 63, 25–34 (2006).
Sowell, E. R. et al. Cortical abnormalities in children and adolescents with attention deficit hyperactivity disorder. Lancet 362, 1699–1707 (2003).
Sowell, E. R. et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb. Cortex 12, 856–865 (2002).
Sowell, E. R. et al. Gray matter thickness abnormalities mapped in children with Tourette Syndrome. Soc. Neurosci. Abstr. 800.15 (2004).
Gogtay, N. et al. Dynamic mapping of cortical brain development in pediatric bipolar illness. Int. Conf. Org. Hum. Brain Mapp. 344 (2004).
Thompson, P. et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 24, 6028–6036 (2004).
Mega, M. S. et al. Sulcal variability in the Alzheimer's brain: correlations with cognition. Neurology 50, 145–151 (1998).
Miller, M. Computational anatomy: shape, growth and atrophy comparison via diffeomorphisms. NeuroImage 23, S19–S33 (2004).
Studholme, C. et al. Deformation tensor morphometry of semantic dementia with quantitative validation. NeuroImage 21, 1387–1398 (2004).
Cannon, T. D. et al. Mapping heritability and molecular genetic associations with cortical features using probabilistic brain atlases: methods and initial applications to schizophrenia. Neuroinformatics 4, 5–19 (2006).
Ballmeier, M. et al. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer's disease using cortical pattern matching: diagnosis and gender effects, NeuroImage 23, 325–335 (2004).
Thompson, P. M., Mega, M. S., Vidal, C., Rapoport, J. L. & Toga, A. W. in Lect. Notes Comput. Sci. 2082, 488–501 (2001).
Thompson, P. M. et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc. Natl Acad. Sci. USA 98, 11650–11655 (2001).
Vidal, C. N. et al. dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch. Gen. Psychiatry 63, 25–34 (2006).
Worsley, K. J. et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 4, 58–73 (1996).
Friston, K. J. Statistical parametric mapping: ontology and current issues. J. Cereb. Blood Flow Metab. 15, 361–370 (1995).
Friston, K. J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1995).
Ashburner, J. & Friston, K. J. Voxel-based morphometry — the methods. NeuroImage 11, 805–821 (2000).
Thompson, P. M. et al. Thinning of the cerebral cortex in HIV/AIDS reflects CD4+ T-lymphocyte decline. Proc. Natl Acad. Sci. 102, 15647–15652 (2005).
Williams, T. H., Gluhbegoric, N. & Jew, J. Y. The human brain: dissections of the real brain. Virtual Hospital, University of Iowa [online], (1997).
Acknowledgements
This work was supported by research grants from the National Institutes of Health (NIH), Roadmap Initiative for Bioinformatics and Computational Biology, National Center for Research Resources, the National Institute of Mental Health (NIMH) and the National Institute of Neurological Disorders and Stroke, and by Human Brain Project grants to the International Consortium for Brain Mapping, funded jointly by NIMH and the National Institute on Drug Abuse and one funded by the National Institute of Aging (NIA). Additional support was provided by the National Institute for Biomedical Imaging and Bioengineering, the National Center for Research Resources and the NIA, the National Library of Medicine and the Biomedical Informatics Research Network (BIRN, http://www.nbirn.net), which is funded by the National Center for Research Resources at the NIH. Other funds came from the German Ministry of Science BMBF, the Helmholtz Association of Research Centres and from various grants from the German Research Foundation DFG and the European Union. We thank the many collaborators and doctoral students in our laboratories. Special thanks to P. Roland from the Karolinska Institute Stockholm for an exciting collaboration of many years, and to N. Palomero-Gallagher for her enthusiasm in the receptor project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Alzheimer's Disease Neuroimaging Initiative
Glossary
- Cartographic approaches
-
Approaches that place brain images from multiple subjects and devices into an anatomical reference system with standardized three-dimensional coordinates (for volumetric images) or two-dimensional spherical or planar coordinates (for cortical regions).
- Cytoarchitecture
-
Subdivisions (named or numbered) of the cerebral cortex, called cytoarchitectonic maps, based on cellular features (size and shape of cells, cell packing density in different cortical layers, width of layers) and identified in cell body-stained specimens.
- Magnetic resonance imaging
-
(MRI). A non-invasive method to obtain images of living tissue. It uses radio-frequency pulses and magnetic field gradients; the principle of nuclear magnetic resonance is used to reconstruct images of tissue characteristics (for example, proton density, water diffusion parameters).
- Multispectral characterization
-
Multispectral imaging devices measure multiple features of an object at each spatial location, such as optical reflectance at different wavelengths, or different relaxometric decay constants (T1 and T2) in MRI.
- Chemoarchitectural maps
-
Differences in the molecular composition of cortical and subcortical brain regions can be assessed using enzyme- or immunohistochemistry, in situ hybridization, receptor autoradiography and so on, revealing subdivisions with distinctive distribution patterns, such as expression of transmitter receptors.
- Diffusion tensor imaging
-
(DTI). A technique developed in the mid-1990s, based on MRI in which diffusion constants of water molecules are measured along many (>6) orientations and diffusion anisotropy is characterized. It is used to visualize the location, orientation and anisotropy of the brain's white matter tracts, and is sensitive to directional parameters of water diffusion in the brain.
- Myeloarchitecture
-
Subdivisions (numbered or named) of the cerebral cortex, called myeloarchitectonic maps, based on features (for example, stria of Gennari in the visual cortex) of myelinization (differential density of myelinated fibres and fibre bundles in different cortical layers), and identified in myelin-stained histological specimens.
- Stereotaxic system
-
A technique or apparatus used in neurosurgery or brain imaging studies to localize a specific anatomical locus using standardized three-dimensional coordinates; used, for example, for directing the tip of a surgical instrument (such as a needle or electrode) to a known location.
- Functional imaging
-
Distinct from structural imaging techniques such as computerized tomography or MRI that assess anatomical structure, functional imaging techniques (for example, positron emission tomography, functional MRI and electroencephalography) are sensitive to physiological processes such as neuronal activation, electromagnetic properties of living tissue, blood flow or metabolism.
- Multimodal association cortices
-
The multimodal association cortices of the parietal and frontal lobes integrate somatosensory, auditory and visual information for higher-order cognitive processing.
- Positron emission tomography
-
A medical imaging technique that uses injected radiolabelled tracer compounds in conjunction with mathematical reconstruction methods to produce a three-dimensional image or map of functional processes in the body, such as glucose metabolism, blood flow or receptor distributions.
- Multimodal microstructural approach
-
An approach to characterize fine-scale anatomy using multiple histological and neurochemical techniques, revealing different aspects of cellular organization or molecular composition.
- Fibre-tracking approaches
-
Using this approach, three-dimensional trajectories of white matter tracts can be reconstructed. The algorithm is based on fibre orientation information obtained from diffusion tensor imaging.
- Probability map
-
A map that depicts the likelihood of a particular feature. It can be used to show how frequently, in percentage, a given anatomical structure is found in a specific location across a population of subjects.
- Cytoarchitectonic probability maps
-
Based on a sample of brains that have been parcellated using cytoarchitectonic criteria; they display the statistical likelihood, or relative frequency, that a particular voxel in stereotaxic space contains a given cytoarchitectonic unit (for example, a cortical area, or a subcortical nucleus).
- Maximum probability maps
-
Summary maps in which each voxel of the three-dimensional space has been assigned to the cytoarchitectonically defined unit with the highest probability. They are calculated on the basis of probability maps, such as cytoarchitectonic probability maps, to generate a map with unambiguously defined borders.
- Voxel
-
The three dimensional equivalent of a pixel. A pixel is a picture element, and a voxel is a volume element.
- T1-weighted images
-
One of most widely used MRI methods, in which the contrast is based on a selection of MRI acquisition parameters, producing an image that weights signal by the relaxometric parameter, T1, of each tissue (that is, the longitudinal relaxation time). In the brain, T1-weighting causes fibre tracts (nerve connections) to appear white, cortex and basal nuclei to appear grey, and cerebrospinal fluid to appear dark.
- Relaxometry
-
The measurement of relaxation parameters in nuclear MRI, such as the longitudinal (T1) or transverse (T2) decay constants that characterize signal decay from excited nuclei. By detecting subtle differences in relaxation times, relaxometry is capable of differentiating various tissue types in the brain.
- Anisotropy map
-
The directional dependency of water diffusion at each point in the brain can be summarized using measures such as fractional anisotropy. High anisotropy values indicate heavily myelinated white matter, whereas decreased anisotropy is often a sign of disease.
- Anisotropic diffusion
-
Diffusion of a substance (for example, water) that is greater in certain preferred directions, such as along the axons of a fibre tract.
- Isotropic diffusion
-
Diffusion of a substance (for example, water) that is uniform in all directions.
- Tensor-valued information
-
Information that can be modelled mathematically as a matrix, or tensor, at each location in an object. Diffusion tensor imaging produces signals with at least six independent parameters at each anatomical point (the diffusion tensor); tensor calculus can then be used to estimate diffusion parameters in any specific direction.
- Partial volume effects
-
This refers to the blurring of intensity differentiations used to classify contributing tissue types (grey matter, white matter and cerebrospinal fluid). It is the results of pixels placed over a region that contains multiple tissue types. The smaller the pixel size the less frequently this is problematic.
Rights and permissions
About this article
Cite this article
Toga, A., Thompson, P., Mori, S. et al. Towards multimodal atlases of the human brain. Nat Rev Neurosci 7, 952–966 (2006). https://doi.org/10.1038/nrn2012
Issue Date:
DOI: https://doi.org/10.1038/nrn2012
This article is cited by
-
A whole-body diffusion MRI normal atlas: development, evaluation and initial use
Cancer Imaging (2023)
-
Temporal dynamics of the neural representation of hue and luminance polarity
Nature Communications (2022)
-
Latent shape image learning via disentangled representation for cross-sequence image registration and segmentation
International Journal of Computer Assisted Radiology and Surgery (2022)
-
Towards an Architecture of a Multi-purpose, User-Extendable Reference Human Brain Atlas
Neuroinformatics (2022)
-
Evolution of Human Brain Atlases in Terms of Content, Applications, Functionality, and Availability
Neuroinformatics (2021)