Key Points
-
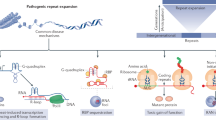
Diseases of unstable repeat expansion are a diverse group of disorders that are caused by the expansion of trinucleotide, tetranucleotide, or pentanucleotide repeat sequences. The position of the repeat sequence determines whether the pathogenic mechanism leads to loss of protein function, altered or enhanced function, or abnormal RNA–protein interactions.
-
Disorders that are caused by loss-of-function mechanisms include the neurodevelopmental fragile X disorders FRAXA and FRAXE and the degenerative disorder Friedreich ataxia. In these disorders repeat expansion results in transcriptional silencing and loss of the gene product.
-
Understanding the roles of FMRP in FRAXA (translational regulation at the synapse), FMR2 in FRAXE (transcription/signalling), and frataxin in FRDA (mitochondrial protein) is providing an insight into pathogenesis and avenues for therapy.
-
Repeat expansion disorders that are caused by altered protein function — the polyglutamine diseases — include several spinocerebellar ataxias, Huntington disease, spinal and bulbar muscular atrophy, and dentatorubral-pallidoluysian atrophy. These disorders share many points of pathogenic convergence, such as protein misfolding and accumulation, but also unique aspects that are determined by the normal function of the disease protein.
-
Mounting evidence highlights the importance of protein context — the protein sequence in which the polygutamine expansion occurs — as a key determinant of pathogenesis, with modulation of function being driven by the expanded polyglutamine tract.
-
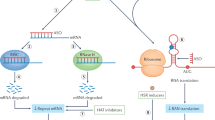
More recently, altered RNA function and/or interactions have been recognized as pathogenic mechanisms in repeat expansion disorders. These alterations underlie dystrophia myotonica 1 and 2 (DM1 and DM2) and fragile X tremor/ataxia syndrome.
-
In DM1 and DM2, the evidence indicates that expanded RNA transcripts lead to the dysregulation of specific RNA-binding proteins; this results in aberrant splicing of several transcripts and a broad, multi-systemic phenotype.
-
In addition to the commonalities within classes of expansion disorders certain similarities also exist between classes. These include importance of normal protein function in protein-mediated disorders, and evidence of protein misfolding in diseases that are caused by both expanded protein and RNA.
-
The convergence of both RNA and protein-mediated diseases on components of the protein quality-control machinery indicates that repeat expansions, whether in the RNA or protein, could elicit a common cellular-response mechanism.
-
Increasing insight into pathogenic mechanisms in these disorders is providing the framework for developing targeted therapies. The late onset of many of the neurodegenerative repeat expansion disorders provides a window of therapeutic opportunity.
Abstract
The list of developmental and degenerative diseases that are caused by expansion of unstable repeats continues to grow, and is now approaching 20 disorders. The pathogenic mechanisms that underlie these disorders involve either loss of protein function or gain of function at the protein or RNA level. Common themes have emerged within and between these different classes of disease; for example, among disorders that are caused by gain-of-function mechanisms, altered protein conformations are central to pathogenesis, leading to changes in protein activity or abundance. In all these diseases, the context of the expanded repeat and the abundance, subcellular localization and interactions of the proteins and RNAs that are affected have key roles in disease-specific phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Verkerk, A. J. et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1991).
La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E. & Fischbeck, K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Liquori, C. L. et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867 (2001).
Matsuura, T. et al. Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10. Nature Genet. 26, 191–194 (2000).
Bennetto, L. & Pennington, B. F. in Fragile X Syndrome Diagnosis, Treatment, and Research (eds Hagerman, R. J. & Cronister, A) 210–248 (Johns Hopkins Univ. Press, Baltimore, 1996).
Merenstein, S. A. et al. Molecular-clinical correlations in males with an expanded FMR1 mutation. Am. J. Med. Genet. 64, 388–394 (1996).
Warren, S. T. & Sherman, S. L. in The Metabolic and Molecular Basis of Inherited Disease Vol. 8 (eds Scriver, C. R. & Sly, W. S.) 1257–1289 (McGraw Hill, New York, 2001).
Hagerman, R. J. et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57, 127–130 (2001).
Corbin, F. et al. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum. Mol. Genet. 6, 1465–1472 (1997).
Li, Z. et al. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 29, 2276–2283 (2001).
Laggerbauer, B., Ostareck, D., Keidel, E. M., Ostareck-Lederer, A. & Fischer, U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum. Mol. Genet. 10, 329–338 (2001).
De Boulle, K. et al. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nature Genet. 3, 31–35 (1993).
Feng, Y. et al. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1, 109–118 (1997).
Bear, M. F., Huber, K. M. & Warren, S. T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002).
Irwin, S. A. et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet. 98, 161–167 (2001).
McBride, S. M. et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45, 753–764 (2005). The investigators use a Drosophila model of fragile X syndrome to show the therapeutic benefit of treatment with mGluR antagonists, providing strong in vivo support of the mGluR theory of fragile X syndrome.
Brown, V. et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107, 477–487 (2001).
Darnell, J. C. et al. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107, 489–499 (2001). References 18 and 19 describe innovative approaches to identify FMRP targets.
Schaeffer, C. et al. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 20, 4803–4813 (2001).
Zhang, Y. Q. et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107, 591–603 (2001).
Xu, K. et al. The fragile X-related gene affects the crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr. Biol. 14, 1025–1034 (2004).
Lee, A. et al. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130, 5543–5552 (2003).
Darnell, J. C. et al. Kissing complex RNAs mediate interaction between the fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 19, 903–918 (2005). The authors identified an RNA target motif for the KH2 domain of FMRP (termed the FMRP kissing complex), showing that the interaction of FMRP with brain polyribosomes occurs through its association with this motif rather than G-quartet RNA motifs.
Caudy, A. A., Myers, M., Hannon, G. J. & Hammond, S. M. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 16, 2491–2496 (2002).
Ishizuka, A., Siomi, M. C. & Siomi, H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 16, 2497–2508 (2002). References 25 and 26 provide some of the first evidence for an association of Drosophila FMRP with the RNAi machinery, supporting the importance of this association for FMRP's role in translational control.
Jin, P. et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nature Neurosci. 7, 113–117 (2004).
Jin, P., Alisch, R. S. & Warren, S. T. RNA and microRNAs in fragile X mental retardation. Nature Cell Biol. 6, 1048–1053 (2004).
Mulley, J. C. et al. FRAXE and mental retardation. J. Med. Genet. 32, 162–169 (1995).
Knight, S. J. et al. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell 74, 127–134 (1993).
Gecz, J., Gedeon, A. K., Sutherland, G. R. & Mulley, J. C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nature Genet. 13, 105–108 (1996).
Gu, Y., Shen, Y., Gibbs, R. A. & Nelson, D. L. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nature Genet. 13, 109–113 (1996).
Nilson, I. et al. Exon/intron structure of the human AF-4 gene, a member of the AF-4/LAF-4/FMR-2 gene family coding for a nuclear protein with structural alterations in acute leukaemia. Br. J. Haematol. 98, 157–169 (1997).
Gecz, J., Bielby, S., Sutherland, G. R. & Mulley, J. C. Gene structure and subcellular localization of FMR2, a member of a new family of putative transcription activators. Genomics 44, 201–213 (1997).
Miller, W. J., Skinner, J. A., Foss, G. S. & Davies, K. E. Localization of the fragile X mental retardation 2 (FMR2) protein in mammalian brain. Eur. J. Neurosci. 12, 381–384 (2000).
Gu, Y. et al. Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J. Neurosci. 22, 2753–2763 (2002).
Su, M. A., Wisotzkey, R. G. & Newfeld, S. J. A screen for modifiers of decapentaplegic mutant phenotypes identifies lilliputian, the only member of the Fragile-X/Burkitt's Lymphoma family of transcription factors in Drosophila melanogaster. Genetics 157, 717–725 (2001).
Wittwer, F., van der Straten, A., Keleman, K., Dickson, B. J. & Hafen, E. Lilliputian: an AF4/FMR2-related protein that controls cell identity and cell growth. Development 128, 791–800 (2001).
Campuzano, V. et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996).
Campuzano, V. et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet. 6, 1771–1780 (1997).
Babcock, M. et al. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276, 1709–1712 (1997).
Foury, F. & Cazzalini, O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett. 411, 373–377 (1997).
Foury, F. Low iron concentration and aconitase deficiency in a yeast frataxin homologue deficient strain. FEBS Lett. 456, 281–284 (1999).
Muhlenhoff, U., Richhardt, N., Ristow, M., Kispal, G. & Lill, R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum. Mol. Genet. 11, 2025–2036 (2002).
Yoon, T. & Cowan, J. A. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J. Am. Chem. Soc. 125, 6078–6084 (2003).
Yoon, T. & Cowan, J. A. Frataxin-mediated iron delivery to ferrochelatase in the final step of heme biosynthesis. J. Biol. Chem. 279, 25943–25946 (2004).
Delatycki, M. B. et al. Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann. Neurol. 45, 673–675 (1999).
Lamarche, J. B., Shapcott, D., Côté, M. & Lemieux, B. in Handbook of Cerebellar Diseases (ed. Lechtenberg, R.) 453–458 (Marcel Dekker, New York, 1993).
Rotig, A. et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nature Genet. 17, 215–217 (1997).
Cossee, M. et al. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219–1226 (2000).
Puccio, H. et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe–S enzyme deficiency followed by intramitochondrial iron deposits. Nature Genet. 27, 181–186 (2001). This study reports the generation and characterization of conditional FRDA mouse models, which recapitulate many features of the human disease. Importantly, it also provides in vivo evidence that mitochondrial iron accumulation only takes place well after the onset of pathology.
Voncken, M., Ioannou, P. & Delatycki, M. B. Friedreich ataxia-update on pathogenesis and possible therapies. Neurogenetics 5, 1–8 (2004).
Wong, A. et al. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum. Mol. Genet. 8, 425–430 (1999).
Chantrel-Groussard, K. et al. Disabled early recruitment of antioxidant defenses in Friedreich's ataxia. Hum. Mol. Genet. 10, 2061–2067 (2001).
Emond, M., Lepage, G., Vanasse, M. & Pandolfo, M. Increased levels of plasma malondialdehyde in Friedreich ataxia. Neurology 55, 1752–1753 (2000).
Seznec, H. et al. Friedreich ataxia: the oxidative stress paradox. Hum. Mol. Genet. 14, 463–474 (2005).
Zoghbi, H. Y. & Orr, H. T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Nakamura, K. et al. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum. Mol. Genet. 10, 1441–1448 (2001).
Watase, K. et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 34, 905–919 (2002).
Yoo, S. Y. et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron 37, 383–401 (2003).
Matilla, A. et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J. Neurosci. 18, 5508–5516 (1998).
Dragatsis, I., Levine, M. S. & Zeitlin, S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nature Genet. 26, 300–306 (2000).
Zuccato, C. et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001).
Ciechanover, A. & Brundin, P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40, 427–446 (2003).
Chan, H. Y., Warrick, J. M., Gray-Board, G. L., Paulson, H. L. & Bonini, N. M. Mechanisms of chaperone suppression of polyglutamine disease: selectivity, synergy and modulation of protein solubility in Drosophila. Hum. Mol. Genet. 9, 2811–2820 (2000).
Cummings, C. J. et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 10, 1511–1518 (2001).
Venkatraman, P., Wetzel, R., Tanaka, M., Nukina, N. & Goldberg, A. L. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol. Cell 14, 95–104 (2004).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin–proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Bennett, E. J., Bence, N. F., Jayakumar, R. & Kopito, R. R. Global impairment of the ubiquitin–proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell 17, 351–365 (2005).
Verhoef, L. G., Lindsten, K., Masucci, M. G. & Dantuma, N. P. Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum. Mol. Genet. 11, 2689–2700 (2002).
Michalik, A. & Van Broeckhoven, C. Proteasome degrades soluble expanded polyglutamine completely and efficiently. Neurobiol. Dis. 16, 202–211 (2004).
Kaytor, M. D., Wilkinson, K. D. & Warren, S. T. Modulating huntingtin half-life alters polyglutamine-dependent aggregate formation and cell toxicity. J. Neurochem. 89, 962–973 (2004).
Bowman, A. B., Yoo, S. Y., Dantuma, N. P. & Zoghbi, H. Y. Neuronal dysfunction in a polyglutamine disease model occurs in the absence of ubiquitin–proteasome system impairment and inversely correlates with the degree of nuclear inclusion formation. Hum. Mol. Genet. 14, 679–691 (2005). The first in vivo study of UPS activity in vulnerable neurons, arguing against a role for UPS impairment in polyglutamine diseases and providing evidence for a protective role of inclusions.
Arrasate, M., Mitra, S., Schweitzer, E. S., Segal, M. R. & Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810 (2004). This is an elegant in vitro study showing that inclusions have a protective role against pathology induced by an N-terminal fragment of huntingtin carrying a polyglutamine repeat expansion.
Slow, E. J. et al. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc. Natl Acad. Sci. USA 102, 11402–11407 (2005). Using a transgenic HD mouse model that expresses a short fragment of huntingtin, this study provides in vivo evidence against the toxicity of inclusions and emphasizes the importance of protein context in pathogenesis.
Ravikumar, B. et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nature Genet. 36, 585–595 (2004).
Jiang, H., Nucifora, F. C. Jr, Ross, C. A. & DeFranco, D. B. Cell death triggered by polyglutamine-expanded huntingtin in a neuronal cell line is associated with degradation of CREB-binding protein. Hum. Mol. Genet. 12, 1–12 (2003).
Tsuda, H. et al. The AXH domain in mammalian/Drosophila ataxin-1 mediates neurodegeneration in spinocerebellar ataxia 1 through its interaction with Gfi-1/Senseless proteins. Cell 122, 1–12 (2005). This study uses genetics and biochemistry to highlight the importance of domains that are outside the polyglutamine tract as key determinants of pathogenesis. It also suggests that the polyglutamine tract exerts toxicity by modulating the function and interactions of the wild-type protein.
Klement, I. A. et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95, 41–53 (1998).
Lin, X., Antalffy, B., Kang, D., Orr, H. T. & Zoghbi, H. Y. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nature Neurosci. 3, 157–163 (2000).
Nucifora, F. C. Jr et al. Interference by huntingtin and atrophin-1 with CBP-mediated transcription leading to cellular toxicity. Science 291, 2423–2428 (2001).
Steffan, J. S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001).
Dunah, A. W. et al. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 296, 2238–2243 (2002).
Li, S. H. et al. Interaction of Huntington disease protein with transcriptional activator Sp1. Mol. Cell. Biol. 22, 1277–1287 (2002).
Steffan, J. S. et al. The Huntington's disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl Acad. Sci. USA 97, 6763–6768 (2000).
McCampbell, A. et al. CREB-binding protein sequestration by expanded polyglutamine. Hum. Mol. Genet. 9, 2197–2202 (2000).
Yu, Z. X., Li, S. H., Nguyen, H. P. & Li, X. J. Huntingtin inclusions do not deplete polyglutamine-containing transcription factors in HD mice. Hum. Mol. Genet. 11, 905–914 (2002).
Schaffar, G. et al. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol. Cell. 15, 95–105 (2004).
Boutell, J. M. et al. Aberrant interactions of transcriptional repressor proteins with the Huntington's disease gene product, huntingtin. Hum. Mol. Genet. 8, 1647–1655 (1999).
Zhang, S., Xu, L., Lee, J. & Xu, T. Drosophila atrophin homolog functions as a transcriptional corepressor in multiple developmental processes. Cell 108, 45–56 (2002).
Tsai, C. C. et al. Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors. Proc. Natl Acad. Sci. USA 101, 4047–4052 (2004).
Palhan, V. B. et al. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc. Natl Acad. Sci. USA 102, 8472–8477 (2005).
McMahon, S. J., Pray-Grant, M. G., Schieltz, D., Yates, J. R. 3rd & Grant, P. A. Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity. Proc. Natl Acad. Sci. USA 102, 8478–8482 (2005).
McCampbell, A. et al. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc. Natl Acad. Sci. USA 98, 15179–15184 (2001).
Hockly, E. et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc. Natl Acad. Sci. USA 100, 2041–2046 (2003).
Ferrante, R. J. et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J. Neurosci. 23, 9418–9427 (2003).
Minamiyama, M. et al. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 13, 1183–1192 (2004). References 94–97 provide evidence for pathogenic polyglutamine-dependent chromatin remodelling and the potential therapeutic use of histone deacetylase inhibitors to reverse this.
Fernandez-Funez, P. et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408, 101–106 (2000).
Shibata, H., Huynh, D. P. & Pulst, S. M. A novel protein with RNA-binding motifs interacts with ataxin-2. Hum. Mol. Genet. 9, 1303–1313 (2000).
Gunawardena, S. et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25–40 (2003).
Szebenyi, G. et al. Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron 40, 41–52 (2003).
Panov, A. V. et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nature Neurosci. 5, 731–736 (2002).
Tang, T. S. et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc. Natl Acad. Sci. USA 102, 2602–2607 (2005).
Chen, H. K. et al. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell 113, 457–468 (2003).
Emamian, E. S. et al. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron 38, 375–387 (2003).
Steffan, J. S. et al. SUMO modification of huntingtin and Huntington's disease pathology. Science 304, 100–104 (2004). References 104–106 highlight the importance of modifications of the host protein in polyglutamine pathogenesis.
Frontali, M. Spinocerebellar ataxia type 6: channelopathy or glutamine repeat disorder? Brain Res. Bull. 56, 227–231 (2001).
Warrick, J. M. et al. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol. Cell 18, 37–48 (2005).
Schmidt, B. J., Greenberg, C. R., Allingham-Hawkins, D. J. & Spriggs, E. L. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology 59, 770–772 (2002).
Takeyama, K. et al. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35, 855–864 (2002).
Katsuno, M. et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002).
Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 69, 385 (1992).
Fu, Y. H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Ranum, L. P., Rasmussen, P. F., Benzow, K. A., Koob, M. D. & Day, J. W. Genetic mapping of a second myotonic dystrophy locus. Nature Genet. 19, 196–198 (1998).
Harper, P. Myotonic Dystrophy Vol. 37 (W.B. Saunders, London, 2001).
Jansen, G. et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nature Genet. 13, 316–324 (1996).
Reddy, S. et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nature Genet. 13, 325–335 (1996).
Klesert, T. R. et al. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nature Genet. 25, 105–109 (2000).
Ranum, L. P. & Day, J. W. Pathogenic RNA repeats: an expanding role in genetic disease. Trends Genet. 20, 506–512 (2004).
Mankodi, A. et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773 (2000).
Mankodi, A. et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 10, 35–44 (2002).
Fardaei, M. et al. Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum. Mol. Genet. 11, 805–814 (2002).
Miller, J. W. et al. Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J. 19, 4439–4448 (2000).
Kanadia, R. N. et al. A muscleblind knockout model for myotonic dystrophy. Science 302, 1978–1980 (2003). The disruption of the mouse Mbnl1 gene reported in this study replicates many of the splicing defects and disease phenotypes of DM1, which clearly implicates the MBNL family in pathogenesis.
Timchenko, N. A. et al. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J. Biol. Chem. 276, 7820–7826 (2001).
Ho, T. H., Bundman, D., Armstrong, D. L. & Cooper, T. A. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet. 14, 1539–1547 (2005). This is an elegant in vitro study that provides evidence that formation of CUG RNA foci in DM1 is separable from MBNL-mediated splicing alterations, and therefore might not be the primary determinant of pathogenesis.
Philips, A. V., Timchenko, L. T. & Cooper, T. A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280, 737–741 (1998).
Savkur, R. S., Philips, A. V. & Cooper, T. A. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nature Genet. 29, 40–47 (2001).
Charlet, B. N. et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell 10, 45–53 (2002).
Jiang, H., Mankodi, A., Swanson, M. S., Moxley, R. T. & Thornton, C. A. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum. Mol. Genet. 13, 3079–3088 (2004). This paper provides some of the first evidence that the RNA-mediated pathogenesis in dystrophia myotonica extends to the CNS through effects on splicing. It also highlights the potential involvement of protein misfolding in diseases that are mediated by aberrant RNA–protein interactions.
Ho, T. H. et al. Muscleblind proteins regulate alternative splicing. EMBO J. 23, 3103–3112 (2004).
Ebralidze, A., Wang, Y., Petkova, V., Ebralidse, K. & Junghans, R. P. RNA leaching of transcription factors disrupts transcription in myotonic dystrophy. Science 303, 383–387 (2004).
Di Prospero, N. A. & Fischbeck, K. H. Therapeutics development for triplet repeat expansion diseases. Nature Rev. Genet. 6, 756–765 (2005).
Jacquemont, S. et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am. J. Hum. Genet. 72, 869–878 (2003).
Greco, C. M. et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125, 1760–1771 (2002).
Tassone, F. et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15 (2000).
Tassone, F., Hagerman, R. J., Chamberlain, W. D. & Hagerman, P. J. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am. J. Med. Genet. 97, 195–203 (2000).
Primerano, B. et al. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA 8, 1482–1488 (2002).
Willemsen, R. et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum. Mol. Genet. 12, 949–959 (2003).
Van Dam, D. et al. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS). Behav. Brain Res. 162, 233–239 (2005).
Jin, P. et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron 39, 739–747 (2003).
Ho, T. H. et al. Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J. Cell Sci. 118, 2923–2933 (2005).
Koob, M. D. et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nature Genet. 21, 379–384 (1999).
Nemes, J. P., Benzow, K. A., Moseley, M. L., Ranum, L. P. & Koob, M. D. The SCA8 transcript is an antisense RNA to a brain-specific transcript encoding a novel actin-binding protein (KLHL1). Hum. Mol. Genet. 9, 1543–1551 (2000).
Mutsuddi, M., Marshall, C. M., Benzow, K. A., Koob, M. D. & Rebay, I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr. Biol. 14, 302–308 (2004).
Holmes, S. E. et al. Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nature Genet. 23, 391–392 (1999).
Holmes, S. E., O'Hearn, E. & Margolis, R. L. Why is SCA12 different from other SCAs? Cytogenet. Genome Res. 100, 189–197 (2003).
Holmes, S. E. et al. A repeat expansion in the gene encoding junctophilin-3 is associated with Huntington disease-like 2. Nature Genet. 29, 377–378 (2001).
Zu, T. et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 24, 8853–8861 (2004).
Yamamoto, A., Lucas, J. J. & Hen, R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 101, 57–66 (2000).
Acknowledgements
The authors wish to thank members of the Zoghbi laboratory and Thai Ho (T. Cooper's laboratory) for critical comments. The authors apologize to anyone whose work they did not cite owing to space constraints. The citations are representative works, and are not inclusive of the many important contributing works for a given topic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
OMIM
dentatorubral-pallidoluysian atrophy
Glossary
- POLYRIBOSOME
-
A string of 80S ribosomes that are bound to an mRNA molecule.
- DENDRITIC SPINES
-
Mushroom-shaped structures on neuronal dendrites that receive synaptic input and have postsynaptic densities. Changes in spine shape are thought to be important for modulating synaptic strength.
- LONG-TERM DEPRESSION
-
A long-lasting decrease in synaptic strength to below baseline levels. It is generally induced in the hippocampus by repetitive low-frequency stimulation. It is thought to result from changes in postsynaptic receptor density, although changes in presynaptic release might also have a role.
- GROUP 1 mGluRs
-
Postsynaptic G-protein coupled receptors for the excitatory neurotransmitter glutamate. These receptors have many roles in synaptic plasticity.
- SYNAPTIC PRUNING
-
The selective elimination of weak synapses during brain development.
- SYNAPTIC PLASTICITY
-
The capacity for alterations in synaptic connections between neurons, including changes in the nature, strength or number of interneuronal connections, which subserves learning and memory.
- G QUARTET
-
A secondary structure observed in G-rich RNA in which four consecutive guanosine residues bind to each other to form an intramolecular stem-loop structure.
- RNA-INDUCED SILENCING COMPLEX
-
A multi-protein complex that functions in RNAi pathways to mediate the cleavage of target RNAs.
- ARGONAUTE PROTEINS
-
A family of proteins that are essential for diverse RNA-silencing pathways.
- DICER PROTEINS
-
A highly conserved family of RNaseIII enzymes that mediate dsRNA cleavage. This produces the small RNAs that direct target silencing in RNAi pathways.
- MICRORNA
-
A form of ssRNA typically 20–25 nucleotides long that is thought to regulate the expression of other genes, either through inhibiting protein translation or degrading a target mRNA transcript through a process that is similar to RNA interference.
- CpG ISLANDS
-
Sequences of at least 200 bp that contain both a GC content that is greater than 50% and a high CpG frequency.
- FERRITIN
-
An iron-storage protein.
- ATAXIA
-
The inability to coordinate movement.
- ACONITASE
-
An enzyme that catalyses the reversible hydration of cis-aconitase to yield citrate or isocitrate. Aconitases are involved in the citric acid cycle.
- IRON–SULPHUR (FE–S) CLUSTERS
-
Co-factor-like species that are common in nature and are involved in many cellular processes, such as electron transfer, catalysis, gene regulation and the sensing of iron and oxygen.
- UBIQUITIN–PROTEASOME SYSTEM
-
A key pathway that is involved in the degradation of intracellular proteins, especially of short-lived regulatory proteins. The system uses an enzymatic cascade that involves conjugation of multiple ubiquitin moieties to a protein substrate and degradation of the tagged protein by the 26S proteasome complex.
- PURKINJE CELLS
-
The neurons that convey the output signals of the cerebellar cortex.
- AUTOPHAGY
-
An intracellular pathway that leads to bulk protein degradation and involves the sequestering of cytosol into vesicles for delivery to a degradative organelle.
- AXOPLASM
-
The intracellular fluid of the axon that facilitates vesicle transport.
- CASPASE
-
One of a family of proteases, activated by proteolytic cleavage, that have an important role in apoptosis and inflammation.
- CALPAINS
-
A family of highly conserved and widely expressed cysteine proteases that are activated by a rise in intracellular calcium levels. They have an important role in apoptosis and in modulating intracellular signals in response to stressful conditions.
- SUMOYLATION
-
The post-translational modification of proteins that involves the covalent attachment of small ubiquitin-like modifier (SUMO) and regulates the interactions of those proteins with other macromolecules.
- P/Q-TYPE CALCIUM CHANNEL
-
A high voltage activated calcium channel found in cerebellar Purkinje cells that is responsible for most of the calcium current in these neurons.
Rights and permissions
About this article
Cite this article
Gatchel, J., Zoghbi, H. Diseases of Unstable Repeat Expansion: Mechanisms and Common Principles. Nat Rev Genet 6, 743–755 (2005). https://doi.org/10.1038/nrg1691
Issue Date:
DOI: https://doi.org/10.1038/nrg1691
This article is cited by
-
RNA structure promotes liquid-to-solid phase transition of short RNAs in neuronal dysfunction
Communications Biology (2024)
-
RNAs undergo phase transitions with lower critical solution temperatures
Nature Chemistry (2023)
-
Rediscovering tandem repeat variation in schizophrenia: challenges and opportunities
Translational Psychiatry (2023)
-
Variations in the poly-histidine repeat motif of HOXA1 contribute to bicuspid aortic valve in mouse and zebrafish
Nature Communications (2023)
-
The length of uninterrupted CAG repeats in stem regions of repeat disease associated hairpins determines the amount of short CAG oligonucleotides that are toxic to cells through RNA interference
Cell Death & Disease (2022)