Key Points
-
Small integrin-binding ligand N-linked glycoproteins (SIBLINGs) are a family of glycophosphoproteins comprising osteopontin (OPN), bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP) and matrix extracellular phosphoglycoprotein (MEPE).
-
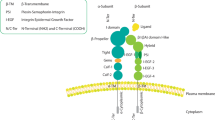
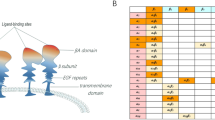
The genes encoding the SIBLINGs are located within a cluster on chromosome 4 and encode soluble, hydrophilic proteins sharing common functional motifs and domains, including an Arg–Gly–Asp (RGD) motif that binds αvβ3 integrin.
-
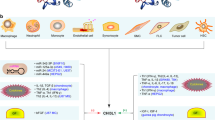
SIBLINGs were initially described as mineralized tissue-associated glycophosphoproteins and were thought to be functionally restricted to these tissues. Recent results show that they are more widely distributed and are expressed in non-mineralized normal tissue, such as metabolically active ductal epithelial cells.
-
Some SIBLINGs activate specific metalloproteinases (MMPs; BSP activates MMP2, OPN, MMP3 and DMP1, MMP9). These three SIBLINGs also bind complement factor H and prevent complement attack.
-
SIBLINGs are overexpressed in many cancers. OPN and, less so, BSP are by far the more widely studied to date and their levels of expression are correlated with tumour aggressiveness. SIBLINGs can be detected in the blood and their level of expression is associated with prognosis.
-
Among SIBLINGs, OPN is involved in almost all steps of tumour progression, including invasion, metastasis and angiogenesis.
-
In vitro and in vivo experimental models demonstrated that interference with SIBLINGs, such as small interfering RNA selective knockdown, has potential anticancer therapeutic value.
-
Identifying the specific roles of SIBLINGs in cancer–stroma interactions and signalling cascades involving growth factor–growth factor receptor and cell–matrix interactions could result in the development of additional and refined strategies for the prevention and treatment of metastases.
Abstract
Numerous components and pathways are involved in the complex interplay between cancer cells and their environment. The family of glycophosphoproteins comprising osteopontin, bone sialoprotein, dentin matrix protein 1, dentin sialophosphoprotein and matrix extracellular phosphoglycoprotein — small integrin-binding ligand N-linked glycoproteins (SIBLINGs) — are emerging as important players in many stages of cancer progression. From their detection in various human cancers to the demonstration of their key functional roles during malignant transformation, invasion and metastasis, the SIBLINGs are proteins with potential as diagnostic and prognostic tools, as well as new therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fisher, L. W., Torchia, D. A., Fohr, B., Young, M. F. & Fedarko, N. S. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 280, 460–465 (2001). Like many other proteins that have multiple binding partners, BSP and OPN are shown by NMR to be unstructured and flexible in solution. The SIBLING family is first defined here based on common exon and intron elements.
Chaplet, M. et al. Expression of dentin sialophospho-protein in human prostate cancer and its correlation with tumor aggressiveness. Int. J. Cancer 118, 850–856 (2006).
Chen, J. et al. Bone sialoprotein promotes tumor cell migration in both in vitro and in vivo models. Connect. Tissue Res. 44 (Suppl. 1), 279–284 (2003).
El-Tanani, M. K. et al. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 17, 463–474 (2006). This is a detailed overview of OPN-mediated cell signalling in relation to cancer progression.
Furger, K. A., Menon, R. K., Tuck, A. B., Bramwell, V. H. & Chambers, A. F. The functional and clinical roles of osteopontin in cancer and metastasis. Curr. Mol. Med. 1, 621–632 (2001).
Sung, V., Stubbs, J. T. 3rd, Fisher, L., Aaron, A. D. & Thompson, E. W. Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the αvβ3 and αvβ5 integrins. J. Cell Physiol. 176, 482–494 (1998).
Tatusova, T. A. & Madden, T. L. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 174, 247–250 (1999).
Fisher, L. W., Whitson, S. W., Avioli, L. V. & Termine, J. D. Matrix sialoprotein of developing bone. J. Biol. Chem. 258, 12723–12727 (1983).
George, A., Sabsay, B., Simonian, P. A. & Veis, A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 268, 12624–12630 (1993).
Feng, J. Q. et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J. Biol. Chem. 273, 9457–9464 (1998).
Oldberg, A., Franzen, A. & Heinegard, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg–Gly–Asp cell-binding sequence. Proc. Natl Acad. Sci. USA 83, 8819–8823 (1986).
Hunter, G. K., Hauschka, P. V., Poole, A. R., Rosenberg, L. C. & Goldberg, H. A. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem. J. 317, 59–64 (1996).
Bellahcene, A. & Castronovo, V. Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull. Cancer 84, 17–24 (1997).
Ogbureke, K. U. & Fisher, L. W. Expression of SIBLINGs and their partner MMPs in salivary glands. J. Dent Res. 83, 664–670 (2004).
Ogbureke, K. U. & Fisher, L. W. Renal expression of SIBLING proteins and their partner matrix metallo-proteinases (MMPs). Kidney Int. 68, 155–166 (2005).
Ogbureke, K. U. & Fisher, L. W. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J. Histochem. Cytochem. 55, 403–409 (2007).
Furger, K. A. et al. β3 integrin expression increases breast carcinoma cell responsiveness to the malignancy-enhancing effects of osteopontin. Mol. Cancer Res. 1, 810–819 (2003).
Rangaswami, H., Bulbule, A. & Kundu, G. C. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 16, 79–87 (2006).
Teramoto, H. et al. Autocrine activation of an osteopontin-CD44–Rac pathway enhances invasion and transformation by H-RasV12. Oncogene 24, 489–501 (2005).
Agnihotri, R. et al. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J. Biol. Chem. 276, 28261–28267 (2001).
Prince, C. W., Dickie, D. & Krumdieck, C. L. Osteopontin, a substrate for transglutaminase and factor XIII activity. Biochem. Biophys. Res. Commun. 177, 1205–1210 (1991).
Higashikawa, F., Eboshida, A. & Yokosaki, Y. Enhanced biological activity of polymeric osteopontin. FEBS Lett. 581, 2697–2701 (2007).
Valverde, P., Tu, Q. & Chen, J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J. Bone Miner. Res. 20, 1669–1679 (2005).
Hur, E. M. et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 8, 74–83 (2007).
Iczkiewicz, J., Jackson, M. J., Smith, L. A., Rose, S. & Jenner, P. Osteopontin expression in substantia nigra in MPTP-treated primates and in Parkinson's disease. Brain Res. 1118, 239–250 (2006).
Rice, J., Courter, D. L., Giachelli, C. M. & Scatena, M. Molecular mediators of αvβ3-induced endothelial cell survival. J. Vasc Res. 43, 422–436 (2006).
Kawamura, K. et al. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin. Diagn. Lab. Immunol. 12, 206–212 (2005).
Lee, J. L. et al. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN–CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 67, 2089–2097 (2007).
Brakebusch, C., Hirsch, E., Potocnik, A. & Fassler, R. Genetic analysis of β1 integrin function: confirmed, new and revised roles for a crucial family of cell adhesion molecules. J. Cell Sci. 110, 2895–2904 (1997).
Katagiri, Y. U. et al. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine–glycine–aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 59, 219–226 (1999).
Christensen, B. et al. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J. Biol. Chem. 282, 19463–19472 (2007).
Pecheur, I. et al. Integrin αvβ3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 16, 1266–1268 (2002).
van der Pluijm, G. et al. Bone sialoprotein peptides are potent inhibitors of breast cancer cell adhesion to bone. Cancer Res. 56, 1948–1955 (1996).
Standal, T. et al. Osteopontin is an adhesive factor for myeloma cells and is found in increased levels in plasma from patients with multiple myeloma. Haematologica 89, 174–182 (2004).
Kulkarni, G. V., Chen, B., Malone, J. P., Narayanan, A. S. & George, A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch. Oral Biol. 45, 475–484 (2000).
Sharp, J. A., Waltham, M., Williams, E. D., Henderson, M. A. & Thompson, E. W. Transfection of MDA-MB-231 human breast carcinoma cells with bone sialoprotein (BSP) stimulates migration and invasion in vitro and growth of primary and secondary tumors in nude mice. Clin. Exp. Metastasis 21, 19–29 (2004).
Khodavirdi, A. C. et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res. 66, 883–888 (2006).
Angelucci, A. et al. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate 59, 157–166 (2004).
Lecrone, V., Li, W., Devoll, R. E., Logothetis, C. & Farach-Carson, M. C. Calcium signals in prostate cancer cells: specific activation by bone-matrix proteins. Cell Calcium 27, 35–42 (2000).
Bell, W. R. The fibrinolytic system in neoplasia. Semin. Thromb. Hemost 22, 459–478 (1996).
Price, J. T. & Thompson, E. W. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin. Ther. Targets. 6, 217–233 (2002).
Hayashi, C. et al. Serum osteopontin, an enhancer of tumor metastasis to bone, promotes B16 melanoma cell migration. J. Cell Biochem. 101, 979–986 (2007).
Khan, S. A. et al. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin. Exp. Metastasis 22, 663–673 (2005).
Caers, J. et al. The involvement of osteopontin and its receptors in multiple myeloma cell survival, migration and invasion in the murine 5T33MM model. Br. J. Haematol. 132, 469–477 (2006). This paper describes possible roles of OPN interactions with integrins and CD44 variants in survival, increasing cell proliferation, inhibiting apoptosis and promoting cell migration, as well as metalloproteinase activity in multiple myeloma cells. It used blocking antibodies (against CD44 and/or αvβ3) to block migration.
Kolb, A. et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol. Ther. 4, 740–746 (2005).
Hu, Z. et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin. Cancer Res. 11, 4646–4652 (2005).
Tuck, A. B. et al. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene 18, 4237–4246 (1999).
Mi, Z., Guo, H., Wai, P. Y., Gao, C. & Kuo, P. C. Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis 27, 1134–1145 (2006).
Das, R., Mahabeleshwar, G. H. & Kundu, G. C. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J. Biol. Chem. 279, 11051–11064 (2004).
Teti, A. et al. Activation of MMP-2 by human GCT23 giant cell tumour cells induced by osteopontin, bone sialoprotein and GRGDSP peptides is RGD and cell shape change dependent. Int. J. Cancer 77, 82–93 (1998).
Rangaswami, H., Bulbule, A. & Kundu, G. C. Nuclear factor inducing kinase: a key regulator in osteopontin- induced MAPK/IκB kinase dependent NF-κB-mediated promatrix metalloproteinase-9 activation. Glycoconj. J. 23, 221–232 (2006).
Rangaswami, H. & Kundu, G. C. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol. Rep. 18, 909–915 (2007).
Fedarko, N. S., Jain, A., Karadag, A. & Fisher, L. W. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 18, 734–736 (2004). This paper shows that specific SIBLINGs bind MMPs as well as release them from inhibition by TIMPs and low-molecular-weight inhibitors. CFH blocks formation of the SIBLING–MMP complexes and can inactivate previously formed complexes and therefore potentially acts as a negative regulator of many SIBLING functions.
Fisher, L. W., Jain, A., Tayback, M. & Fedarko, N. S. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin. Cancer Res. 10, 8501–8511 (2004). This paper explores SIBLINGs expression in various cancer types on cancer patient microarray. The degree of correlation between a SIBLING and its partner MMP expression within a given cancer type was also addressed.
Karadag, A., Ogbureke, K. U., Fedarko, N. S. & Fisher, L. W. Bone sialoprotein, matrix metallo-proteinase 2, and αvβ3 integrin in osteotropic cancer cell invasion. J. Natl Cancer Inst. 96, 956–965 (2004).
Karadag, A., Fedarko, N. S. & Fisher, L. W. Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res. 65, 11545–11552 (2005).
Takafuji, V., Forgues, M., Unsworth, E., Goldsmith, P. & Wang, X. W. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene 26, 6361–6371 (2007).
Mangala, L. S., Arun, B., Sahin, A. A. & Mehta, K. Tissue transglutaminase-induced alterations in extracellular matrix inhibit tumor invasion. Mol. Cancer 4, 33 (2005).
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Rev. Cancer 3, 453–458 (2003).
Oates, A. J., Barraclough, R. & Rudland, P. S. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene 13, 97–104 (1996).
Wai, P. Y. & Kuo, P. C. The role of Osteopontin in tumor metastasis. J. Surg. Res. 121, 228–241 (2004).
Adwan, H., Bauerle, T. J. & Berger, M. R. Downregulation of osteopontin and bone sialoprotein II is related to reduced colony formation and metastasis formation of MDA-MB-231 human breast cancer cells. Cancer Gene Ther. 11, 109–120 (2004). This was the first paper to demonstrate that downregulating the expression of OPN or BSP in cancer cells inhibits the formation of bone metastases in vivo.
Nemoto, H. et al. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J. Bone Miner. Res. 16, 652–659 (2001).
Bellahcene, A., Merville, M. P. & Castronovo, V. Expression of bone sialoprotein, a bone matrix protein, in human breast cancer. Cancer Res. 54, 2823–2826 (1994). The first immunohistochemical demonstration of ectopic BSP expression in human breast cancer tumours.
Waltregny, D. et al. Prognostic value of bone sialoprotein expression in clinically localized human prostate cancer. J. Natl Cancer Inst. 90, 1000–1008 (1998).
Bellahcene, A. et al. Expression of bone sialoprotein in human lung cancer. Calcif. Tissue Int. 61, 183–188 (1997).
Bellahcene, A. et al. Bone sialoprotein mRNA and protein expression in human multiple myeloma cell lines and patients. Br. J. Haematol. 111, 1118–1121 (2000).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Zhang, J. H. et al. Over-expression of bone sialoprotein enhances bone metastasis of human breast cancer cells in a mouse model. Int. J. Oncol. 23, 1043–1048 (2003).
Zhang, J. H. et al. Bone sialoprotein promotes bone metastasis of a non-bone-seeking clone of human breast cancer cells. Anticancer Res. 24, 1361–1368 (2004).
Koeneman, K. S., Yeung, F. & Chung, L. W. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 39, 246–261 (1999). A detailed study of the 'osteomimicry hypothesis' to explain the propensity of prostate cancer cells to grow and establish in the bone microenvironment.
Zayzafoon, M., Abdulkadir, S. A. & McDonald, J. M. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J. Biol. Chem. 279, 3662–3670 (2004).
Bellahcene, A. et al. Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res. Treat 101, 135–148 (2007).
Aggarwal, B. B., Shishodia, S., Sandur, S. K., Pandey, M. K. & Sethi, G. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 72, 1605–1621 (2006).
McKee, M. D. & Nanci, A. Secretion of Osteopontin by macrophages and its accumulation at tissue surfaces during wound healing in mineralized tissues: a potential requirement for macrophage adhesion and phagocytosis. Anat. Rec. 245, 394–409 (1996).
O'Regan, A. W. et al. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J. Immunol. 162, 1024–1031 (1999).
Weber, G. F. & Cantor, H. The immunology of η-1/osteopontin. Cytokine Growth Factor Rev. 7, 241–248 (1996).
Giachelli, C. M., Lombardi, D., Johnson, R. J., Murry, C. E. & Almeida, M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 152, 353–358 (1998).
Koh, A. et al. Role of osteopontin in neutrophil function. Immunology 122, 466–475 (2007).
Wai, P. Y. et al. Osteopontin inhibits macrophage nitric oxide synthesis to enhance tumor proliferation. Surgery 140, 132–140 (2006).
Shiba, H. et al. Macrophage inflammatory protein-3α and β-defensin-2 stimulate dentin sialophosphoprotein gene expression in human pulp cells. Biochem. Biophys. Res. Commun. 306, 867–871 (2003).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Volanakis, J. E. in The Human Complement System in Health and Disease (eds Volankis, J. E. & Frank, M. M.) 9–32 (Marcel Dekker, New York, 1998).
Fedarko, N. S., Fohr, B., Robey, P. G., Young, M. F. & Fisher, L. W. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J. Biol. Chem. 275, 16666–16672 (2000).
Jain, A., Karadag, A., Fohr, B., Fisher, L. W. & Fedarko, N. S. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H's cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J. Biol. Chem. 277, 13700–13708 (2002).
Nam, J. S. et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor β in a mouse model of breast cancer. Cancer Res. 66, 6327–6335 (2006). This paper reports a direct association between TGFβ pro-metastatic activity and BSP expression in breast cancer cells.
Brooks, P. C., Clark, R. A. & Cheresh, D. A. Requirement of vascular integrin αvβ3 for angiogenesis. Science 264, 569–571 (1994).
Cui, R. et al. Abrogation of the interaction between osteopontin and αvβ3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer 57, 302–310 (2007). A demonstration in a mouse model that disruption of the interaction of osteopontin with the αvβ3 integrin has therapeutic potential to diminish tumour progression.
Tang, H. et al. Inhibition of osteopontin would suppress angiogenesis in gastric cancer. Biochem. Cell Biol. 85, 103–110 (2007).
Bellahcene, A. et al. Bone sialoprotein mediates human endothelial cell attachment and migration and promotes angiogenesis. Circ. Res. 86, 885–891 (2000). The first study to show BSP has angiogenesis-promoting activity using in vitro and in vivo assays.
Chakraborty, G., Jain, S. & Kundu, G. C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 68, 152–161 (2008).
Robey, P. G. Vertebrate mineralized matrix proteins: structure and function. Connect. Tissue Res. 35, 131–136 (1996).
Giachelli, C. M. Inducers and inhibitors of biomineralization: lessons from pathological calcification. Orthod. Craniofac. Res. 8, 229–231 (2005).
Carlinfante, G. et al. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin. Exp. Metastasis 20, 437–444 (2003).
Tunio, G. M., Hirota, S., Nomura, S. & Kitamura, Y. Possible relation of osteopontin to development of psammoma bodies in human papillary thyroid cancer. Arch. Pathol. Lab. Med. 122, 1087–1090 (1998).
Steitz, S. A. et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 161, 2035–2046 (2002).
Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell Biochem. 99, 1233–1239 (2006).
Sato, M. et al. Transcriptional regulation of osteopontin gene in vivo by PEBP2αA/CBFA1 and ETS1 in the skeletal tissues. Oncogene 17, 1517–1525 (1998).
Javed, A. et al. runt homology domain transcription factors (RUNX, CBFA, and AML) mediate repression of the bone sialoprotein promoter: evidence for promoter context-dependent activity of Cbfa proteins. Mol. Cell Biol. 21, 2891–2905 (2001).
Fen, J. Q. et al. Dentin matrix protein 1, a target molecule for CBFA1 in bone, is a unique bone marker gene. J. Bone Miner. Res. 17, 1822–1831 (2002).
Chen, S. et al. Differential regulation of dentin sialophosphoprotein expression by RUNX2 during odontoblast cytodifferentiation. J. Biol. Chem. 280, 29717–29727 (2005).
Blyth, K., Cameron, E. R. & Neil, J. C. The RUNX genes: gain or loss of function in cancer. Nature Rev. Cancer 5, 376–387 (2005).
Pratap, J. et al. Regulatory roles of RUNX2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 25, 589–600 (2006).
Inman, C. K. & Shore, P. The osteoblast transcription factor RUNX2 is expressed in mammary epithelial cells and mediates osteopontin expression. J. Biol. Chem. 278, 48684–48689 (2003).
Barnes, G. L. et al. Osteoblast-related transcription factors RUNX2 (CBFA1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 63, 2631–2637 (2003).
Colla, S. et al. Human myeloma cells express the bone regulating gene RUNX2/CBFA1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Leukemia 19, 2166–2176 (2005).
Agrawal, D. et al. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J. Natl Cancer Inst. 94, 513–521 (2002).
Wai, P. Y. et al. ETS-1 and RUNX2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J. Biol. Chem. 281, 18973–18982 (2006).
El-Tanani, M. K. et al. BRCA1 suppresses osteopontin-mediated breast cancer. J. Biol. Chem. 281, 26587–26601 (2006).
Packer, L. et al. Osteopontin is a downstream effector of the PI3-kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinogenesis 27, 1778–1786 (2006).
Kim, M. S. et al. Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to enhance the motility of human glioma cells. Cancer Res. 65, 686–691 (2005).
Samant, R. S. et al. Breast cancer metastasis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NFκB activation. Mol. Cancer 6, 6 (2007).
Senger, D., Asch, B., Smith, B., Perruzzi, C. & Dvorak, H. A secreted phosphoprotein marker for neoplastic transformation of both epithelial and fibroblastic cells. Nature 302, 714–715 (1983).
Senger, D. R., Wirth, D. F. & Hynes, R. O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 16, 885–893 (1979).
Bellahcene, A. et al. Ectopic expression of bone sialoprotein in human thyroid cancer. Thyroid 8, 637–641 (1998).
Detry, C. et al. Detection of bone sialoprotein in human (pre)neoplastic lesions of the uterine cervix. Calcif. Tissue Int. 73, 9–14 (2003).
Kayed, H. et al. Effects of bone sialoprotein on pancreatic cancer cell growth, invasion and metastasis. Cancer Lett. 245, 171–183 (2007).
Riminucci, M. et al. Coexpression of bone sialoprotein (BSP) and the pivotal transcriptional regulator of osteogenesis, CBFA1/RUNX2, in malignant melanoma. Calcif. Tissue Int. 73, 281–289 (2003).
Ogbureke, K. U. et al. Up-regulation of SIBLING proteins and correlation with cognate MMP expression in oral cancer. Oral Oncol. 43, 920–932 (2007).
Chaplet, M. et al. Dentin matrix protein 1 is expressed in human lung cancer. J. Bone Miner. Res. 18, 1506–1512 (2003).
Bucciarelli, E. et al. Low dentin matrix protein 1 expression correlates with skeletal metastases development in breast cancer patients and enhances cell migratory capacity in vitro. Breast Cancer Res. Treat. 105, 95–104 (2007).
Rowe, P. S. et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67, 54–68 (2000).
Agrawal, D. et al. Osteopontin identified as colon cancer tumor progression marker. C. R. Biol. 326, 1041–1043 (2003).
Rohde, F. et al. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int. J. Cancer (2007).
Bao, L. H., Sakaguchi, H., Fujimoto, J. & Tamaya, T. Osteopontin in metastatic lesions as a prognostic marker in ovarian cancers. J. Biomed. Sci. 14, 373–381 (2007).
Matusan, K., Dordevic, G., Stipic, D., Mozetic, V. & Lucin, K. Osteopontin expression correlates with prognostic variables and survival in clear cell renal cell carcinoma. J. Surg. Oncol. 94, 325–331 (2006).
Sakaguchi, H., Fujimoto, J., Hong, B. L. & Tamaya, T. Clinical implications of osteopontin in metastatic lesions of uterine cervical cancers. Cancer Lett. 247, 98–102 (2007).
Rudland, P. S. et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 62, 3417–3427 (2002). This paper demonstrates on a large collection of breast cancer specimens that OPN expression levels are an independent prognostic indicator that correlates with reduced patient survival.
Forootan, S. S. et al. Prognostic significance of osteopontin expression in human prostate cancer. Int. J. Cancer 118, 2255–2261 (2006).
Bache, M. et al. Immunohistochemical detection of osteopontin in advanced head-and-neck cancer: prognostic role and correlation with oxygen electrode measurements, hypoxia-inducible-factor-1α-related markers, and hemoglobin levels. Int. J. Radiat. Oncol. Biol. Phys. 66, 1481–1487 (2006).
Celetti, A. et al. Overexpression of the cytokine osteopontin identifies aggressive laryngeal squamous cell carcinomas and enhances carcinoma cell proliferation and invasiveness. Clin. Cancer Res. 11, 8019–8027 (2005).
Guarino, V. et al. Osteopontin is overexpressed in human papillary thyroid carcinomas and enhances thyroid carcinoma cell invasiveness. J. Clin. Endocrinol. Metab. 90, 5270–5278 (2005).
Donati, V. et al. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin. Cancer Res. 11, 6459–6465 (2005).
Pan, H. W. et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer 98, 119–127 (2003).
Bellahcene, A., Menard, S., Bufalino, R., Moreau, L. & Castronovo, V. Expression of bone sialoprotein in primary human breast cancer is associated with poor survival. Int. J. Cancer 69, 350–353 (1996).
Papotti, M. et al. Bone sialoprotein is predictive of bone metastases in resectable non-small-cell lung cancer: a retrospective case-control study. J. Clin. Oncol. 24, 4818–4824 (2006). This study identifies BSP expression as a predictive factor of bone metastasis development in patients with resectable NSCLC.
Fedarko, N. S., Jain, A., Karadag, A., Van Eman, M. R. & Fisher, L. W. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin. Cancer Res. 7, 4060–4066 (2001).
Scatena, M., Liaw, L. & Giachelli, C. M. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb. Vasc Biol. 27, 2302–2309 (2007).
Bramwell, V. H. et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin. Cancer Res. 12, 3337–3343 (2006).
Ramankulov, A., Lein, M., Kristiansen, G., Loening, S. A. & Jung, K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate 67, 330–340 (2007).
Ramankulov, A. et al. Elevated plasma osteopontin as marker for distant metastases and poor survival in patients with renal cell carcinoma. J. Cancer Res. Clin. Oncol. 133, 643–652 (2007).
Chang, Y. S. et al. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer 57, 373–380 (2007).
Wu, C. Y. et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut 56, 782–789 (2007).
Petrik, D. et al. Plasma osteopontin is an independent prognostic marker for head and neck cancers. J. Clin. Oncol. 24, 5291–5297 (2006).
Zhang, H. et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 132, 709–717 (2006).
Koopmann, J. et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 13, 487–491 (2004).
Diel, I. J. et al. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin. Cancer Res. 5, 3914–3919 (1999).
Woitge, H. W. et al. Serum bone sialoprotein as a marker of tumour burden and neoplastic bone involvement and as a prognostic factor in multiple myeloma. Br. J. Cancer 84, 344–351 (2001).
Jung, K. et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int. J. Cancer 111, 783–791 (2004).
Ito, T. et al. An inducible short-hairpin RNA vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivo. Clin. Cancer Res. 12, 1308–1316 (2006).
Wai, P. Y. et al. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis 26, 741–751 (2005).
de Fougerolles, A., Vornlocher, H. P., Maraganore, J. & Lieberman, J. Interfering with disease: a progress report on siRNA-based therapeutics. Nature Rev. Drug Discov. 6, 443–453 (2007).
Lien, S. & Lowman, H. B. Therapeutic anti-VEGF antibodies. Handb. Exp. Pharmacol. 181, 131–150 (2008).
Ye, Q. H. et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nature Med. 9, 416–423 (2003). This paper combines the demonstration that OPN is a diagnostic and prognostic marker for hepatocellular carcinoma and works as an effective target in antibody-based treatment.
Bauerle, T. et al. Characterization of a rat model with site-specific bone metastasis induced by MDA-MB-231 breast cancer cells and its application to the effects of an antibody against bone sialoprotein. Int. J. Cancer 115, 177–186 (2005).
Weber, G. F. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim. Biophys. Acta 1552, 61–85 (2001).
Brooks, P. C. et al. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79, 1157–1164 (1994).
Melquist, J. J. et al. Conditionally replicating adenovirus-mediated gene therapy in bladder cancer: an orthotopic in vivo model. Urol. Oncol. 24, 362–371 (2006).
Qin, C., Baba, O. & Butler, W. T. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral Biol. Med. 15, 126–136 (2004).
Sreenath, T. et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 278, 24874–24880 (2003).
Senger, D. R., Perruzzi, C. A., Papadopoulos, A. & Tenen, D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim. Biophys. Acta 996, 43–48 (1989).
Patarca, R., Wei, F. Y., Singh, P., Morasso, M. I. & Cantor, H. Dysregulated expression of the T cell cytokine Eta-1 in CD4−8− lymphocytes during the development of murine autoimmune disease. J. Exp. Med. 172, 1177–1183 (1990). An early report on the role of OPN Eta-1 in immune dysregulation. Increased OPN expression was associated with a chronic immune response and autoimmune disease.
Shiraga, H. et al. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc. Natl Acad. Sci. USA 89, 426–430 (1992).
Brown, L. F. et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol. Biol. Cell 3, 1169–1180 (1992).
Terasawa, M. et al. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J. Bone Miner. Metab. 22, 430–438 (2004).
Shen, Q. & Christakos, S. The vitamin D receptor, RUNX2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J. Biol. Chem. 280, 40589–40598 (2005).
Kim, H. J. et al. Okadaic acid stimulates osteopontin expression through de novo induction of AP-1. J. Cell Biochem. 87, 93–102 (2002).
Roca, H., Phimphilai, M., Gopalakrishnan, R., Xiao, G. & Franceschi, R. T. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J. Biol. Chem. 280, 30845–30855 (2005).
Hassan, M. Q. et al. HOXA10 controls osteoblastogenesis by directly activating bone regulatory and phenotypic genes. Mol. Cell Biol. 27, 3337–3352 (2007).
Nakayama, Y. et al. Insulin-like growth factor-I increases bone sialoprotein (BSP) expression through fibroblast growth factor-2 response element and homeodomain protein-binding site in the proximal promoter of the BSP gene. J. Cell Physiol. 208, 326–335 (2006).
Pang, J. et al. Upregulation of dentin matrix protein 1 promoter activities by core binding factor α1 in human dental pulp stem cells. Biochem. Biophys. Res. Commun. 357, 505–510 (2007).
Narayanan, K., Ramachandran, A., Hao, J. & George, A. Transcriptional regulation of dentin matrix protein 1 (DMP1) by AP-1 (c-fos/c-jun) factors. Connect. Tissue Res. 43, 365–371 (2002).
Chen, S. et al. Regulation of the cell type-specific dentin sialophosphoprotein gene expression in mouse odontoblasts by a novel transcription repressor and an activator CCAAT-binding factor. J. Biol. Chem. 279, 42182–42191 (2004).
Oyama, Y., Akuzawa, N., Nagai, R. & Kurabayashi, M. PPARγ ligand inhibits osteopontin gene expression through interference with binding of nuclear factors to A/T-rich sequence in THP-1 cells. Circ. Res. 90, 348–355 (2002).
Lamour, V. et al. Runx2- and histone deacetylase 3-mediated repression is relieved in differentiating human osteoblast cells to allow high bone sialoprotein expression. J. Biol. Chem. 282, 36240–36249 (2007).
Galler, K. M. et al. A novel role for Twist-1 in pulp homeostasis. J. Dent Res. 86, 951–955 (2007).
Chen, B., Piel, W. H., Gui, L., Bruford, E. & Monteiro, A. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 86, 627–637 (2005).
Guo, W. & Giancotti, F. G. Integrin signalling during tumour progression. Nature Rev. Mol. Cell Biol. 5, 816–826 (2004).
Coppola, D. et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin. Cancer Res. 10, 184–190 (2004).
Ang, C., Chambers, A. F., Tuck, A. B., Winquist, E. & Izawa, J. I. Plasma osteopontin levels are predictive of disease stage in patients with transitional cell carcinoma of the bladder. BJU Int. 96, 803–805 (2005).
Sulzbacher, I., Birner, P., Trieb, K., Lang, S. & Chott, A. Expression of osteopontin and vascular endothelial growth factor in benign and malignant bone tumors. Virchows Arch. 441, 345–349 (2002).
Cogan, G. et al. Analysis of human bone sialoprotein in normal and pathological tissues using a monoclonal antibody (BSP 1.2 mab). Connect. Tissue Res. 45, 60–71 (2004).
Valabrega, G. et al. ErbB2 and bone sialoprotein as markers for metastatic osteosarcoma cells. Br. J. Cancer 88, 396–400 (2003).
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature Genet. 38, 1310–1315 (2006).
MacDougall, M., Dong, J. & Acevedo, A. C. Molecular basis of human dentin diseases. Am. J. Med. Genet. A 140, 2536–2546 (2006).
Saitoh, Y., Kuratsu, J., Takeshima, H., Yamamoto, S. & Ushio, Y. Expression of osteopontin in human glioma. Its correlation with the malignancy. Lab. Invest. 72, 55–63 (1995).
Bellahcene, A. & Castronovo, V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am. J. Pathol. 146, 95–100 (1995).
Rodrigues, L. R., Teixeira, J. A., Schmitt, F. L., Paulsson, M. & Lindmark-Mansson, H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol. Biomarkers Prev. 16, 1087–1097 (2007).
Wong, Y. F. et al. Genome-wide gene expression profiling of cervical cancer in Hong Kong women by oligonucleotide microarray. Int. J. Cancer 118, 2461–2469 (2006).
Eschrich, S. et al. Molecular staging for survival prediction of colorectal cancer patients. J. Clin. Oncol. 23, 3526–3535 (2005).
Gaumann, A. et al. Osteopontin expression in primary sarcomas of the pulmonary artery. Virchows Arch. 439, 668–674 (2001).
Ue, T. et al. Co-expression of osteopontin and CD44v9 in gastric cancer. Int. J. Cancer 79, 127–132 (1998).
Matusan, K., Dordevic, G., Mozetic, V. & Lucin, K. Expression of osteopontin and CD44 molecule in papillary renal cell tumors. Pathol. Oncol. Res. 11, 108–113 (2005).
Somerman, M. J., Berry, J. E., Khalkhali-Ellis, Z., Osdoby, P. & Simpson, R. U. Enhanced expression of αv integrin subunit and osteopontin during differentiation of HL-60 cells along the monocytic pathway. Exp. Cell Res. 216, 335–341 (1995).
Flamant, S. et al. Osteopontin is upregulated by BCR-ABL. Biochem. Biophys. Res. Commun. 333, 1378–1384 (2005).
Chambers, A. F. et al. Osteopontin expression in lung cancer. Lung Cancer 15, 311–323 (1996).
Nagai, S. et al. Comprehensive gene expression profile of human activated TH1- and TH2-polarized cells. Int. Immunol. 13, 367–376 (2001).
Saeki, Y. et al. Enhanced production of osteopontin in multiple myeloma: clinical and pathogenic implications. Br. J. Haematol. 123, 263–270 (2003).
Geissinger, E., Weisser, C., Fischer, P., Schartl, M. & Wellbrock, C. Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen. Cancer Res. 62, 4820–4828 (2002).
Zhou, Y. et al. Osteopontin expression correlates with melanoma invasion. J. Invest. Dermatol. 124, 1044–1052 (2005).
Tiniakos, D. G., Yu, H. & Liapis, H. Osteopontin expression in ovarian carcinomas and tumors of low malignant potential (LMP). Hum. Pathol. 29, 1250–1254 (1998).
Tozawa, K. et al. Osteopontin expression in prostate cancer and benign prostatic hyperplasia. Urol. Int. 62, 155–158 (1999).
Hotte, S. J., Winquist, E. W., Stitt, L., Wilson, S. M. & Chambers, A. F. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer 95, 506–512 (2002).
Debucquoy, A. et al. Molecular responses of rectal cancer to preoperative chemoradiation. Radiother Oncol. 80, 172–177 (2006).
Acknowledgements
The work of A.B. and V.C. is supported by grants from the National Fund for Scientific Research (Belgium), the Centre Anti-Cancéreux of the University of Liège and the European Commission through METABRE Contract CEE LSHC-CT-2004-503049. The work of K.O. is supported by National Institute of Dental and Craniofacial Research/National Institutes of Health (NIH) Grant K23 017791-01A1, Medical College of Georgia Research Institute Grant STP 00105W005, and the Wendy Will Case Cancer Fund. The research of L.W.F. is supported by the Intramural Research Program of the NIH, Department of Health and Human Services. The work of N.S.F. is supported by grants from the National Cancer Institute (R21CA87311 and R01 CA113865) and from the Department of Defense Congressionally Directed Medical Research Program (W81XWH-04-1-0844 and BC010478).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Integrin
-
Integrins are a large family of heterodimeric cell surface adhesion receptors that bind extracellular matrix and cell surface ligands. They promote stable interactions between cells and their environment and mediate intracellular signalling.
- Dentin
-
The main, calcareous part of a tooth, beneath the enamel and surrounding the pulp chamber and root canals.
- RGD motif
-
A tripeptide, Arg–Gly–Asp (RGD), found in numerous proteins that support cell adhesion. A subset of the integrins recognize the RGD motif within their ligands, the binding of which mediates both cell–substratum and cell–cell interactions.
- Hydroxyapatite crystals
-
The principal inorganic constituent of bone matrix and teeth, imparting rigidity to these structures, and consisting of hydrated calcium phosphate, Ca5(PO4)3 OH.
- Metabolically active normal duct
-
Epithelia, such as that of the kidney nephrons, that alter the tonicity of the fluid they process in the course of normal physiology. The kidney nephrons process isotonic urine into the voided hypotonic urine.
- Type 0 introns
-
Introns that disrupt an open reading frame between codon junctions and therefore permit any splicing combination to other type 0 exons without causing frameshifts.
- Metabolically passive normal duct
-
Epithelia, such as that of the lacrimal gland ducts, that do not alter the tonicity of the fluid they process in the course of normal physiology. Tears from the lacrimal acini are isotonic and are secreted unchanged through the duct system.
- CD44
-
A family of cell surface signal-transducing glycoproteins involved in cell–cell interactions, cell adhesion and migration. CD44s bind hyaluronan, a high-molecular-mass polysaccharide found in the extracellular matrix, and a variety of extracellular as well as cell surface ligands. CD44 exists in multiple spliced forms and shows a high variability in glycosylation.
- Transglutaminases
-
A family of enzymes that catalyse the crosslinking of proteins at a glutamine in one chain with lysine in another chain. Although the family members have different structures, they share an active site (Tyr–Gly–Gln–Cys–Trp) and strict Ca2+ dependence.
- Osteoclast
-
A cell that breaks down mineralized bone and is responsible for bone resorption.
- Dental pulp cells
-
Cells that comprise the soft tissue forming the inner structure of a tooth and containing nerves and blood vessels as well as possibly dentin stem cells.
- Osteotropic
-
Describes tumours that metastasize preferentially to the skeleton.
- Bone lesions
-
Lytic lesions are areas of the bone marked by destruction, whereas sclerotic lesions are areas of the bone marked by thickening or hardening. A mixed lytic and sclerotic lesion exhibits facets of both resorption (destruction) and thickening (formation).
- Opsonization
-
The process whereby opsonins (antibodies or complement proteins) make an invading cell or microorganism more susceptible to phagocytosis by binding to its surface.
- Chicken chorioallantoic membrane assay
-
A biological assay using the well-vascularized chorioallantoic membrane of the chicken egg to evaluate the biological activity of pro- and anti-angiogenic factors.
- Renal osteodystrophy
-
A bone disease characterized by softening and fibrous degeneration of bone and the formation of cysts in bone tissue, caused by chronic renal failure.
- Eccrine sweat ducts
-
These ducts transport sweat to the surface of the skin and are involved in evaporative cooling.
- Oncogenic hypophosphataemic osteomalacia
-
Osteomalacia (softening of the bones) resulting from renal phosphate wasting and low serum 1, 25-dihydroxy vitamin D secondary to the presence of a tumour of which complete resection results in rapid resolution of the symptoms and signs.
Rights and permissions
About this article
Cite this article
Bellahcène, A., Castronovo, V., Ogbureke, K. et al. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer 8, 212–226 (2008). https://doi.org/10.1038/nrc2345
Issue Date:
DOI: https://doi.org/10.1038/nrc2345
This article is cited by
-
De novo identification of maximally deregulated subnetworks based on multi-omics data with DeRegNet
BMC Bioinformatics (2022)
-
Osteopontin in autoimmune disorders: current knowledge and future perspective
Inflammopharmacology (2022)
-
PHGDH heterogeneity potentiates cancer cell dissemination and metastasis
Nature (2022)
-
The Role of DMP1 in CKD-MBD
Current Osteoporosis Reports (2021)
-
DMP-1 promoter-associated antisense strand non-coding RNA, panRNA-DMP-1, physically associates with EGFR to repress EGF-induced squamous cell carcinoma migration
Molecular and Cellular Biochemistry (2021)