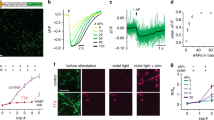
Abstract
Genetically encoded fluorescent probes have become indispensable tools in the biological sciences. Studies of synaptic vesicle recycling have been facilitated by a group of GFP-derived probes called pHluorins. These probes exploit changes in pH that accompany exocytosis and recapture of synaptic vesicles. Here we describe how these synaptic tracers can be used in rodent hippocampal neurons to monitor the synaptic vesicle cycle in real time and to obtain mechanistic insights about it. Synapses can be observed in living samples using a wide-field fluorescence microscope and a cooled charge-coupled device camera. A simple specimen chamber allows electrical stimulation of synapses to evoke exocytosis in a precisely controlled manner. We present protocols to measure various parameters of the synaptic vesicle cycle. This technique can be easily adapted to study different classes of synapses from wild-type and mutant mice. Once cultured neurons expressing synaptopHluorin are available, the whole procedure should take about 2 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
De Paola, V. et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875 (2006).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Kasthuri, N. & Lichtman, J.W. Structural dynamics of synapses in living animals. Curr. Opin. Neurobiol. 14, 105–111 (2004).
Keller-Peck, C.R. et al. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic nice. Neuron 31, 381–394 (2001).
Lendvai, B., Stern, E.A., Chen, B. & Svoboda, K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000).
Miesenbock, G., De Angelis, D.A. & Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescence proteins. Nature 394, 192–195 (1998).
Jahn, R., Lang, T. & Sudhof, T.C. Membrane fusion. Cell 112, 519–533 (2003).
Murthy, V.N. & De Camilli, P. Cell biology of the presynaptic terminal. Annu. Rev. Neurosci. 26, 701–728 (2003).
Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Moulder, K.L. & Mennerick, S. Reluctant vesicles contribute to the total readily releasable pool in glutamatergic hippocampal neurons. J. Neurosci. 25, 3842–3850 (2005).
Rizzoli, S.O. & Betz, W.J. The structural organization of the readily releasable pool of synaptic vesicles. Science 303, 2037–2039 (2004).
Rosenmund, C. & Stevens, C.F. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, 1197–1207 (1996).
Schikorski, T. & Stevens, C.F. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 4, 391–395 (2001).
Sankaranarayanan, S., De Angelis, D., Rothman, J.E. & Ryan, T.A. The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 79, 2199–2208 (2000).
Granseth, B., Odermatt, B., Royle, S. & Lagnado, L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773–786 (2006).
Fernandez-Alfonso, T., Kwan, R. & Ryan, T.A. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron 51, 179–186 (2006).
Wienisch, M. & Klingauf, J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat. Neurosci. 9, 1019–1027 (2006).
Voglmaier, S.M. et al. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71–84 (2006).
Ashby, M.C., Maier, S.R., Nishimune, A. & Henley, J.M. Lateral diffusion drives constitutive exchange of AMPA receptor at dendritic spines and is regulated by spine morphology. J. Neurosci. 26, 7046–7055 (2006).
Jacob, T.C. et al. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J. Neurosci. 25, 10469–10478 (2005).
Pelkey, K.A., Yuan, X., Lavezzari, G., Roche, K.W. & McBain, C.J. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology published online 14 August 2006 (doi: 10.1016/j.neuropharm.2006.07.020).
Ng, M. et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463–474 (2002).
Bozza, T., McGann, J.P., Mombaerts, P. & Wachowiak, M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron 42, 9–21 (2004).
Samuel, A.D.T., Silva, R. & Murthy, V.N. Synaptic activity of the AFD neurons in Caenorhabditis elegans correlates with thermotactic memory. J. Neurosci. 23, 373–376 (2003).
Kim, J. et al. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron 40, 151–165 (2003).
Li, Z. et al. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc. Natl. Acad. Sci. 102, 6131–6136 (2005).
Araki, R. et al. Transgenic mouse lines expressing synaptopHluorin in hippocampus and cerebellar cortex. Genesis 42, 53–60 (2005).
Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 71, 3–9 (1997).
Jiang, M. & Chen, G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat. Protocols 1, 695–700 (2006).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Sankaranarayanan, S. & Ryan, T.A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2, 197–204 (2000).
Li, Z. & Murthy, V.N. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron 31, 593–605 (2001).
Schikorski, T. & Stevens, C.F. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 17, 5858–5867 (1997).
Harata, N., Ryan, T.A., Smith, S.J., Buchanan, J. & Tsien, R.W. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1-43 photoconversion. Proc. Natl. Acad. Sci. USA 98, 12748–12753 (2001).
Newton, A.J., Kirchhausen, T. & Murthy, V.N. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc. Natl. Acad. Sci. U S A. 103, 17955–17960 (2006).
Atluri, P.P. & Ryan, T.A. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J. Neurosci. 26, 2313–2320 (2006).
Yudowski, G.A., Puthenveedu, M.A. & von Zastrow, M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat. Neurosci. 9, 622–627 (2006).
Dittman, J.S. & Kaplan, J.M. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc. Natl. Acad. Sci. 103, 11399–11404 (2006).
Yu, D., Ponomarev, A. & Davis, R.L. Altered representation of the spatial code for odors after olfactory classical conditioning: memory trace formation by synaptic recruitment. Neuron 42, 437–449 (2004).
Poskanzer, K.E., Marek, K.W., Sweeney, S.T. & Davis, G.W. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature 426, 559–563 (2003).
Reiff, D.F. et al. In vivo performance of genetically encoded indicators of neural activity in flies. J. Neurosci. 25, 4766–4778 (2005).
Sturman, D.A., Shakiryanova, D., Hewes, R.S., Deitcher, D.L. & Levitan, E.S. Nearly neutral secretory vesicles in Drosophila nerve terminals. Biophys. J. 90, L45–L47 (2006).
McGann, J.P. et al. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron 48, 1039–1053 (2005).
Wachowiak, M. et al. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J. Neurophysiol. 94, 2700–2712 (2005).
Gandhi, S.P. & Stevens, C.F. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423, 607–613 (2003).
Sankaranarayanan, S. & Ryan, T.A. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat. Neurosci. 4, 129–136 (2001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Note
Protocol for preparing dissociated rodent hippocampal neuron cultures. (PDF 100 kb)
Supplementary Video 1
A typical experiment with neurons obtained from a transgenic mouse line in which a subset of neurons express synaptopHluorin. Fluorescence increases as neurotransmitter is released and spH is exposed to the extracellular space during neuronal stimulation. When stimulation ceases, the fluorescence decays back to baseline due to endocytosis and reacidification of synaptic vesicles. (AVI 3729 kb)
Supplementary Video 2
A typical experiment with neurons obtained from rat hippocampus, where synaptopHluorin expression is achieved by transient transfection. Fluorescence increases as neurotransmitter is released and spH is exposed to the extracellular space during neuronal stimulation. When stimulation ceases, the fluorescence decays back to baseline due to endocytosis and reacidification of synaptic vesicles. (AVI 3112 kb)
Rights and permissions
About this article
Cite this article
Burrone, J., Li, Z. & Murthy, V. Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nat Protoc 1, 2970–2978 (2006). https://doi.org/10.1038/nprot.2006.449
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.449
This article is cited by
-
Hydroxynorketamine, but not ketamine, acts via α7 nicotinic acetylcholine receptor to control presynaptic function and gene expression
Translational Psychiatry (2024)
-
Aberrant activity of mitochondrial NCLX is linked to impaired synaptic transmission and is associated with mental retardation
Communications Biology (2021)
-
CDH2 mutation affecting N-cadherin function causes attention-deficit hyperactivity disorder in humans and mice
Nature Communications (2021)
-
Aβ1-16 controls synaptic vesicle pools at excitatory synapses via cholinergic modulation of synapsin phosphorylation
Cellular and Molecular Life Sciences (2021)
-
Ammonium chloride alters neuronal excitability and synaptic vesicle release
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.