Abstract
A single sub-anesthetic dose of ketamine, a short-acting NMDA receptor blocker, induces a rapid and prolonged antidepressant effect in treatment-resistant major depression. In animal models, ketamine (24 h) reverses depression-like behaviors and associated deficits in excitatory postsynaptic currents (EPSCs) generated in apical dendritic spines of layer V pyramidal cells of medial prefrontal cortex (mPFC). However, little is known about the effects of ketamine on basal dendrites. The basal dendrites of layer V cells receive an excitatory input from pyramidal cells of the basolateral amygdala (BLA), neurons that are activated by the stress hormone CRF. Here we found that CRF induces EPSCs in PFC layer V cells and that ketamine enhanced this effect through the mammalian target of rapamycin complex 1 synaptogenic pathway; the CRF-induced EPSCs required an intact BLA input and were generated primarily in basal dendrites. In contrast to its detrimental effects on apical dendritic structure and function, chronic stress did not induce a loss of CRF-induced EPSCs in basal dendrites, thereby creating a relative imbalance in favor of amygdala inputs. The effects of ketamine were complex: ketamine enhanced apical EPSC responses in all mPFC subregions, anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) but enhanced CRF-induced EPSCs only in AC and PL—responses were unchanged in IL, a critical area for suppression of stress responses. We propose that by restoring the strength of apical inputs relative to basal amygdala inputs, especially in IL, ketamine would ameliorate the hypothesized disproportional negative influence of the amygdala in chronic stress and major depression.
Similar content being viewed by others
INTRODUCTION
Sub-anesthetic doses of ketamine, a short-acting NMDA channel blocker, are known to induce acute (1 to 2 h) psychotomimetic effects in humans that model various positive and negative aspects of schizophrenia (see the study by Krystal et al (1994)) and serves as a paradigm for therapeutics (see the study by Meltzer et al (2013)). Surprisingly, despite its adverse acute effects, ketamine produces an antidepressant effect in treatment resistant depressed patients, which begins shortly after the initial psychotomimetic effects subside (2 to 4 h), peaking at ~24 h and lasting a week or more before gradually fading (Berman et al, 2000; Krystal et al, 2013; Zarate et al, 2013). Similarly, in a chronic stress animal model of depression, a single sub-anesthetic dose of ketamine produces a robust antidepressant effect that also peaks within 24 h and lasts more than a week (Li et al, 2011). Studies in both naïve and stressed animals show that ketamine induces a transient activation of the mammalian target of rapamycin complex 1 (TORC1) pathway in the medial prefrontal cortex (mPFC), triggering dendritic translation of synaptic proteins that are then incorporated into stable synaptic spines in apical dendrites that can persist a week or longer (Li et al, 2010; Li et al, 2011). Such stable structural changes can explain how the antidepressant responses can persist long after the ketamine is cleared from the body (Duman and Aghajanian, 2012). In parallel with the ketamine-induced increase in apical dendritic spines (synaptogenesis), there is a robust and long-lasting increase in the frequency and amplitude of excitatory postsynaptic currents (EPSCs) induced by 5-HT or hypocretin (orexin) recorded from layer V pyramidal cells in the mPFC, indicating that the newly generated spines are functional (Li et al, 2010). The 5-HT-activated inputs have been identified as a small subset of cortico-cortical projections (Weisstaub et al, 2006) while the hypocretin-activated inputs arise from thalamic midline/intralaminar nuclei (Lambe and Aghajanian, 2003), which selectively target the apical dendritic field, demonstrating that diverse inputs to apical dendrites are enhanced by ketamine. Ketamine rapidly reverses the deficit in EPSC responses to 5-HT and hypocretin (Li et al, 2011) that is caused by chronic stress (Liu and Aghajanian, 2008).
While the above studies demonstrate that ketamine can reverse the stress-induced decline in apically targeted inputs to layer V pyramidal cells, it is not known whether ketamine can strengthen synaptic inputs to the basal dendrites of these cells. A possible stress-sensitive input to basal dendrites comes from anterograde tracing that demonstrates a major monosynaptic input projecting from the basolateral amygdala nucleus (BLA) to the basal dendrites of mPFC layer V cells (Bacon et al, 1996; Gabbott et al, 2012). Also, a direct BLA input to layer II pyramidal cells has been shown by anterograde optogenetic methods (Little and Carter, 2013). The main cortically projecting excitatory neurons of the BLA are glutaminergic pyramidal cells. Whole cell recordings in the mPFC of brain slices show that BLA pyramidal neurons are directly depolarized by the application of the stress hormone CRF acting via the CRF1 receptor (Giesbrecht et al, 2010; Van Pett et al, 2000). In vivo studies show that local injection of CRF into the BLA preferentially activates pyramidal cells rather than GAD65-expressing interneurons (Rostkowski et al, 2013). These results raise the possibility that the terminals of the BLA neurons may also express surface CRF1 receptors and thus could similarly be depolarized by CRF. Potentially, such a depolarization could cause glutamate release and generate EPSCs in basal dendrites in mPFC, as we previously found for 5-HT- and hypocretin-activated glutaminergic inputs to apical dendrites (Aghajanian and Marek, 1997; Lambe and Aghajanian, 2003; Liu and Aghajanian, 2008).
In preliminary unpublished studies conducted mostly in the prelimbic (PL) and anterior cingulate (AC) subregions of mPFC, we found that CRF was able to induce EPSCs in layer V pyramidal cells and that this effect was enhanced by ketamine. Beyond repeating these pilot observations, the purpose of this study was to determine (1) if CRF-induced EPSCs are mediated by the CRF1 receptor and if the enhancement of CRF-induced EPSCs by ketamine is dependent on the mTORC1 pathway as previously found for apical dendritic inputs; (2) if CRF-activated inputs emanate from the BLA; (3) if CRF inputs are targeted to the basal dendrites; (4) if chronic stress reduces synaptic responses to CRF as it does in the case of 5-HT-activated apical inputs; and finally (5) if ketamine-enhanced CRF responses differ between the PL and infralimbic (IL), a possibility suggested by different and functionally disparate subsets of BLA pyramidal cells projecting to these subregions of mPFC (Senn et al, 2014).
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 275–350 g were pair-housed and maintained in standard conditions with a 12-h light/dark cycle and ad libitum access to food and water. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
Drug Administration and Surgical Procedures
Drugs given in vivo are as follow: (±)-ketamine hydrochloride, Hospira (Lake Forest, IL); drug stock solutions were dissolved in 0.9% saline immediately before use and injected intraperitoneally (ip). To test the effect of rapamycin, a selective blocker of the mTORC1 synaptogenic pathway, to ketamine rats were implanted with guide cannula (22GA) into the lateral ventricles (coordinates from bregma: −0.9 mm anterior/posterior (AP), −1.5 mm medial/lateral (ML), −3.3 mm dorsal/ventral (DV) from dura) or medial prefrontal cortex (coordinates from bregma: +3.2 mm AP, ±1.0 mm ML, −3.5 mm DV from dura). The surgical procedures were carried out under the anesthesia of Nembutal (ip 55 mg/kg). Postoperative care consisted in perisurgerical administration of carprofen (5 mg/kg) and topical triple antibiotic during which animals carried a dummy cannula. After a 7-day recovery period, rapamycin (100 nmol in 2 μL for i.c.v. infusion, and 10 nmol in 1 μL for PFC infusion), with an injection cannula (26GA) protruding 0.5 mm beyond the guide cannula 30 min before drug injections. The dose of ketamine was chosen based on previous studies showing its effectiveness in activating mTORC1 signaling; the dose of rapamycin was based on previous studies demonstrating its effectiveness in blocking mTORCI activation (Li et al, 2010; 2011. The injection cannula stayed in the guide cannula for 1 min after infusions. In vitro, CRF, NBI 29 143, and CNQX were from Tocris (Ellisville, MO); 5-hydroxytryptamine creatinine sulfate (5-HT) was from Sigma; and hypocretin 2 (orexin B) was from American Peptide. All drug solutions were perfused at known concentrations through a stopcock assembly.
Chronic Unpredictable Stress (CUS) Procedure
Animals were exposed to a variable sequence of mild and unpredictable stressors for 21 days, a procedure which we have found produces depressive-like behavioral changes (Banasr et al, 2007; Banasr and Duman, 2008). A total of 10 different stressors were used (2 stressors per day). The stressors included rotation on a shaker, placement in a 4 °C ambient, lights off for 3 h (10AM to 1PM), lights on overnight, strobe light overnight, aversive odor, 45° tilted cages, food and water deprivation, crowded housing and isolation housing.
BLA Lesioning Procedures
Under pentobarbital anesthesia (50 mg/kg), male Sprague-Dawley rats (190–210 g) were injected with 0.3 μL of either 40 mg/ml NMDA (Sigma) or 0.9% saline at 0.1 μl/min. Each animal received both treatments; the left side was injected with NMDA, and the right side was injected with saline. The coordinates for the BLA were −2.7 mm, +/−4.8 mm, and −8.1 mm relative to Bregma. The injector needle remained in place for 3 min following the injection to allow for diffusion. NMDA was prepared fresh each day and diluted in sterile water. After surgery, rats were given carprofen (5 mg/kg) as an analgesic and 5 ml of 0.9% saline to prevent fluid loss. Rats were killed 3 weeks following the microinjection procedure. Nissl staining and light microscopy were performed on sections containing the amygdala to confirm placements and lesioning.
Brain Slice Preparation and Electrophysiological Recordings
Brain slices were prepared as previously described (Liu and Aghajanian, 2008; Li et al, 2011). Briefly, 1 day after drug treatment, rats were anesthetized (chloral hydrate, 400 mg/kg, ip) and brains were removed. Coronal slices 400-μm thick were cut in ice-cold sucrose–ACSF from a block of tissue containing the mPFC with an oscillating-blade tissue slicer (Leica VT 1000S; GMI). Slices were placed in a submerged recording chamber; bath temperature was then raised slowly to 32 °C. The standard ACSF (pH 7.35), equilibrated with 95% O2/5% CO2, contained 128 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgSO4, 24 mM NaHCO3, 1.25 mM NaH2PO4, and 10 mM d-glucose. There was recovery period of 1–2 h before recording.
Pyramidal neurons in layer V were patched under visual control using infrared differential interference contrast (IR/DIC) microscopy ( × 60 IR lens; Olympus, Center Valley, PA). Patch pipettes (3–5 MΩ) were pulled from glass tubing with a Flaming-Brown Horizontal Puller (Sutter, Novato, CA). The pipette solution contained: 115 mM K gluconate, 5 mM KCl, 2 mM MgCl2, 2 mM Mg-ATP, 2 mM Na2ATP, 10 mM Na2-phosphocreatine, 0.4 mM Na2GTP, and 10 mM HEPES, pH 7.33. Neurobiotin (0.3%) was added to the pipette solution to mark cells for later processing and imaging. Whole-cell recordings were made with an Axoclamp-2B amplifier (Molecular Devices, Sunnyvale, CA). The output signal was low-pass-filtered at 3 KHz and digitized at 15 kHz; data were acquired by pClamp 9.2/Digidata 1320 software (Molecular Devices). Series resistance, monitored throughout the experiment, was usually between 4 and 8 MΩ. To minimize series resistance errors, cells were discarded if series resistance rose above 10 MΩ. Postsynaptic currents were in the continuous single-electrode voltage-clamp mode (3000 Hz low-pass filter) clamped near resting potential (~75±5 mV). Known concentrations of drugs in ACSF were applied through a stopcock arrangement (~4 ml/min), reaching slice within 7–10 s. Electrophysiological data was displayed off-line with Clampfit software of pClamp 9.2 (Axon Instruments). Analysis of EPSCs from each 10-s block of 1-s sweeps was with MiniAnalysis software (Synaptosoft, Fort Lee, NJ).
Spine Density Analysis
After completion of recording, slices were transferred to 4% paraformaldehyde (0.1 M phosphate buffer) and stored overnight at 4 °C. Slices were then processed with Streptavidin conjugated to Alexa 594 (1 : 1000) for visualization of labeled cells. Labeled neurons within layer V of PL and IL mPFC were imaged with a two-photon Ti:sapphire laser scanning system (810 nm; Mai Tai, Spectra Physics, Mountain View, CA) coupled to direct detection Radiance 2000 BioRad laser scanner (Zeiss Micromaging, Thornwood, NY) mounted on an Olympus BX50WI microscope, using a × 60 (0.9 numerical aperture) water-immersion objective. Spine analysis was sampled from the apical tuft and basal dendrites. The length of dendritic segments was determined within the 3D matrix of each Z-stack by using Neurolucida 10.2 (MicroBrightField, Williston, VT). Automated spine density analysis was by the Autospine module of Neurolucida Explorer (version 10.2) on raw image stacks (2–5 optical sections (1 μm)). Results were expressed as spine density per 10 μm.
Analysis and Statistics
Electrophyiological data were displayed off-line with Clampfit software of pClamp 9.2. Analysis of EPSCs from each 10-s block of (1 s) sweeps was performed with MiniAnalysis software. For statistical analysis, unless otherwise noted, we used two-tailed unpaired Student’s t-tests (for comparison of two groups), one-way analysis of variance followed by t-test comparison (for three groups).
RESULTS
Ketamine Enhances CRF-Induced EPSCs; Block by AMPA, CRF1, or mTORC1 Antagonists
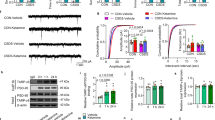
Previous studies have shown that pretreatment with systemic ketamine (24 h) produces a rapid increase in 5-HT- and hypocretin-induced EPSCs in mPFC layer V cells, associated with an increase in spines in apical dendrites (Li et al, 2010). Also, in preliminary studies, we found that CRF was able to induce EPSCs in layer V pyramidal cells. Following up on these pilot experiments in a larger sample of cells, we found that CRF induced EPSCs in ~50% of the cells tested, primarily in the AC and PL subregions; the greatest responses were in thick-tufted cells that had hyperpolarization-activated inward currents (Ih; S1). Moreover, the CRF-induced EPSCs were blocked completely by CNQX (10 μM), a selective AMPA receptor antagonist (Figure 1a). We also found that a selective CRF1 receptor antagonist, NBI29143 at 1 μM, almost completely blocked the CRF-induced EPSCs (Figure 1b, n=7 p<0.01). We next examined CRF responses in animals administered ketamine 24 h earlier. As in the case of 5-HT, ketamine enhanced CRF-induced EPSCs in layer V pyramidal cells (Figure 1c, n=15, p<0.01); the mTORC1 blocker rapamycin completely prevented ketamine enhancement of CRF-induced EPSCs (Figure 1c, n=7), indicating the effect was dependent on activation of the mTORC1 synaptogenic pathway.
CRF-induced EPSCs in mPFC in layer V cells: block by AMPA or CRF1 antagonists and enhanced by ketamine via mTORC1 pathway. (a): Representative traces showing CRF-induced EPSCs (300 μM) recorded from a layer V pyramidal neuron are blocked by the selective AMPA receptor antagonist, CNQX 10 μM. (b): traces showing almost complete block of CRF-induced EPSCs by the CRF1 receptor antagonist, NBI29143 (1 μM); bar graph below showing summary data (mean±SEM, **p<0.01). (c): Bar graph summarizes the EPSC frequency of four groups neurons showing an enhancement of CRF-induced EPSC frequency in brain slices 24 h following pretreatment with ketamine (10 mg/kg, ip); the enhancement by ketamine is totally blocked by pretreatment with rapamycin (i.c.v). Results are given as mean±SEM (N=7 cells, *p<0.05, **p<0.01; ##<0.01, comparison with rapamycin group). EPSC, excitatory postsynaptic current; ip, intraperitoneal; mPFC, medial prefrontal cortex; mTORC1, mammalian target of rapamycin complex 1; NS, not significant.
CRF-Induced EPSCs of Layer V Pyramidal Cells are Reduced by ~50% by Excitotoxin Lesioning of BLA
The source of the input mediating the CRF-induced EPSCs is unknown. One promising possibility is the BLA, which strongly expresses CRF1 receptors (Van Pett et al, 2000), and provides a major excitatory input to mPFC layer V pyramidal neurons. Accordingly, we investigated whether CRF-induced EPSCs depend on the integrity of BLA inputs. Because most of BLA–mPFC projections are ipsilateral, we placed unilateral excitotoxin lesions in the BLA, using both the intact side and normal animals as controls. As shown in Figure 2a, Nissl stained coronal sections from a BLA-lesioned rat illustrate that the lesioned side of the BLA had prominent neuronal loss. Figure 2b shows summary data for the EPSC response before and during CRF application in both the normal control group (baseline, 9.3±1.3; CRF, 15±1.6; n=4 animals, 22 cells, p<0.01) and in the experimental group (9.3±1.2 and 15.8±2 Hz on the vehicle/nonlesioned side (n=29, p< 0.01) vs 7.3±1.4 and 10.4±1.5 Hz, on the lesioned side (n=27, p>0.05; Figure 2b). Note, the frequency of CRF-induced EPSCs was reduced by ~50% on the lesioned side compared with the non-lesioned side (p<0.05), and the CRF response in the vehicle/nonlesioned side was essentially identical to that in the normal controls. These results show that unilateral excitotoxin-induced lesions of the BLA produce a substantial reduction of EPSC responses to CRF recorded in layer V pyramidal cells in ipsilateral mPFC. The sources of the inputs responsible for the remaining CRF-induced are not known but could result partly from incompleteness of the lesions of the BLA, particularly in the anterior–posterior direction.
BLA lesions reduced CRF-induced EPSC frequency of ipsilateral layer V pyramidal cells in mPFC. (a) Representative images of Nissl stained coronal sections taken 2 weeks after excitotoxin injections (20 mg NMDA) into BLA to allow for cell degeneration. (a) Low magnification image ( × 5) of non-lesion side (right side of animal) 2 weeks after intra-BLA infusion with saline. (b) Low magnification image ( × 5) shows lesion side (left side of the animal) 2 weeks after intra-BLA infusion NMDA (40 mg/ml, 0.3 μl). Images below (c and d) show higher magnification views of control and lesioned ( × 20). Note that the neurotoxic lesions of BLA show prominent neuron loss. (b) Summary of data showing unilateral BLA lesion reduced CRF-induced EPSC frequency in ipsilaterally mPFC layer V pyramidal cells. The results are given in mean±SEM *p<0.05, **p<0.01 compared with baselines. #p<0.05 one tail compared between non-lesion side and lesion side with CRF treatment. Note that non-lesion side is essentially identical with control response to CRF; also note the small non-significant reduction in baseline EPSCs on the lesion side. BLA, basolateral amygdale; EPSC, excitatory postsynaptic current; mPFC, medial prefrontal cortex.
Reduction in CRF-Induced EPSCs and Spine Density in the Basal Dendritic Field in mPFC After BLA Lesions
While Gabbott et al (2012) demonstrated that BLA pyramidal cells send projections to basal dendrites of layer V cells of the mPFC, possible projections to apical field could not be assessed because the apical tuft could not be visualized by their labeling technique. Therefore, we compared responses to bath-applied CRF, 5-HT, and hypocretin in neighboring cells, one with a surgical knife cut disconnecting the apical tuft near the bifurcation of the apical trunk and the other with its apical tuft intact (Figure 3a). Because the cut was quite distant from the site of recording, basic electrophysiological properties recorded at the soma (eg, resting membrane potential, spike amplitude) were not affected (data not shown). Summary data shows similar CRF-induced EPSC frequency in both groups (n=9, p>0.05), but a significant decrease of apically generated 5-HT- and Hcrt-induced EPSC frequency in the severed apical tuft group vs control (n=9; P<0.01 for 5-HT) and (n=9; P<0.05 for hcrt). These results indicate that the distal apical dendritic field does not contribute significantly to the CRF response, allowing us to focus on basal dendrites in subsequent experiments. Sholl analyses of basal dendritic arborization revealed that there was no significant reduction in dendritic complexity between 10 and 200 μm from the soma on the BLA lesioned and non-lesioned side (Figure 3b). On the other hand, we found a significant decrease in spine density in BLA damaged side (Figures 4c, p<0.001), which suggests that CRF-induced ESPCs are mainly associated with a reduction of spine density in the basal field.
BLA lesions did not affect the total basal branch length of the layer V pyramidal cells, but reduced spine density. (a) Left image, 3D Z-stack projections showing a pair of neighboring layer V pyramidal cells, one intact and the other with its apical tuft detached. Right, disconnecting the apical tuft did not interfere with the frequency of CRF-induced EPSCs but markedly reduced apically generated 5-HT and hcrt responses *p<0.05, **p<0.01. (b) Sholl analyses of basal dendritic structure showing no significant change in basal dendritic length between lesion side and non-lesion side between 10 and 200 μm from soma. (c) Two-photon images of basal branch segments from recorded mPFC layer V pyramidal neurons filled with neurobiotin and post-hoc staining with streptavidin conjugated to Alexa 594; bar graph on right, summary data showing BLA lesion reduced the basal dendritic spine density (***p<0.001) of layer pyramidal cells in the mPFC. BLA, basolateral amygdale; EPSC, excitatory postsynaptic current; mPFC, medial prefrontal cortex.
Chronic unpredictable stress (CUS) did not decrease CRF-induced EPSCs in layer V pyramidal cells whereas it sharply reduced apically directed 5-HT-induced EPSCs. (a) Examples of whole cell voltage clamp recording traces in layer V pyramidal cells taken from a slice of a non-stressed control or CUS animal; note the lack of effect of CUS CRF-induced EPSCs in contrast with the reduction in 5-HT-induced EPSCs. (b) Summary data (n=13) showing that CUS did not decrease the frequency of CRF-induced EPSC compared with controls (*p<0.05, **p<0.01, #p<0.05). EPSC, excitatory postsynaptic current; NS, not significant.
The Effects of Chronic Stress Exposure on CRF-Induced EPSCs
Previously, we found that CUS exposure decreased the expression levels of synaptic proteins, spine number, and the frequency/amplitude of synaptic currents induced by 5-HT in apical dendrites of layer V pyramidal neurons in the mPFC. These deficits were rapidly reversed by ketamine (Li et al, 2011). This CUS paradigm also decreases sucrose preference, a measure of anhedonia, which is a core symptom of depression (Li et al, 2011). Here we tested whether the frequencies of CRF-induced EPSCs were similarly reduced by the same 21-day CUS exposure. Unexpectedly, we found that the frequency of EPSCs induced by CRF did not significantly differ between the CUS and control animals: EPSC frequencies before and during application of CRF were 7±1.4 and 10.8±1.7 Hz, respectively, in the control group (n=11) and 5.6±1.2 and 10±1.5 Hz, respectively, in CUS group (n=14, p>0.05) (Figure 4). However, the frequency of 5-HT-induced EPSCs showed the expected decrease by CUS: EPSC frequency before and during application of 5-HT, were 6.1±1.6 and 18.9±2.7 Hz (n=10), respectively, in the control group and 7.1±1.3 and 12.2±1.8 Hz (n=14), respectively, in CUS group (p<0.05) (Figure 4b). These results are consistent with the previous finding that CUS leads to apical dendrite atrophy but generally does not affect basal dendrites.
Ketamine Enhances PL but not IL CRF-Induced EPSCs
Recent dual retrograde tracing studies have shown that different subsets of BLA pyramidal cells innervate the PL vs IL subregions of mPFC and subserve contrasting behavioral roles with respect to fear expression and extinction memory (Senn et al, 2014). Given their different roles in behavior and the fact that different subsets of BLA pyramidal cells project to PL and IL, we investigated possible differences in the effect of ketamine on CRF responses in these two subregions. This ketamine treatment paradigm increases the number and function of spine synapses in layer V neurons and produces an antidepressant behavioral response in several different animal models (Li et al, 2010; 2011). Whole cell recording indicated that ketamine enhanced the frequency of CRF-induced EPSCs in PL (4.2±1 Hz for control, 8.6±1.3 Hz for ketamine, n=15, p<0.05) but not IL (3.8±1.3 Hz for control, 1.8±1 Hz for ketamine, n=15, p>0.05, Figure 5a). Next, we examined the influence of ketamine on spine number by two-photon laser imaging of the prelabeled layer V pyramidal neurons in mPFC. Consistent with previous results, ketamine significantly enhanced spine density of apical dendrites in both PL and IL (Figures 5b, p<0.05), but in basal dendrites ketamine only enhanced spine density in PL (6.2±0.2 μm for control, 7.5±0.2 μm for ketamine, p<0.01), but not IL (Figure 5c), which parallels the differential induction of EPSC frequency in these two PFC subregions.
Ketamine pretreatment (10 mg/kg, 24 h) increased the CRF-induced EPSC frequency and the number of basal dendritic spines in layer V pyramidal cells in PL/pregenual AC but not in IL. (a) Summary data (n=15) showing that ketamine increased CRF-induced EPSC frequency in PL/AC (ketamine, 8.6±1.3 and control, 4.2±1 Hz (p=0.016; n=15) but not in IL. (b) Summary data from apical tuft dendritic spine density analysis showing similar increase for both PL/AC and IL (mean±SEM (n=10; *p<0.05, **p<0.01). (c) Summary data from basal dendritic spine density analysis (mean±SEM, n=15, *p<0.05, **p<0.01). Note ketamine enhanced both PL and IL apical dendritic spine density but only enhanced PL basal branch spine density, which is consistent with the observation that ketamine only increased PL EPSCs. (d) Schematic diagrams showing distribution of cells recorded in this study. Each dot represents one cell (control, open circles; ketamine pretreatment, closed circles). AC, anterior cingulate; EPSC, excitatory postsynaptic current; IL, infralimbic; PL, prelimbic.
DISCUSSION
The main findings of this study are that (1) the stress responsive hormone CRF induces CRF1 receptor-dependent EPSCs in mPFC layer V pyramidal cells; (2) pretreatment with rapamycin, a blocker of the mTORC1 synaptogenic pathway, prevents the effects of ketamine on CRF-induced EPSCs in basal dendrites, as previously found for 5-HT-induced EPSCs in apical dendrites (Li et al, 2010); (3) the BLA is a major source for the CRF-activated inputs; (4) chronic stress does not reduce CRF-induced EPSCs, consistent with the general finding that pyramidal cell basal dendrites are resistant to stress-induced atrophy (Magariños and McEwen, 1995; Cook and Wellman, 2004; Radley et al, 2004; Liu and Aghajanian, 2008); and (5) ketamine (24 h) enhances CRF-induced EPSCs in conjunction with an increase in the density of basal dendritic synaptic spines in PL but not IL subregions of mPFC, while 5-HT induces EPSCs and/or spine density in apical dendrites in all subregions including IL. The preferential synaptic atrophy produced by chronic stress in apical dendrites but not the basal field thus creates a relative imbalance favoring the amygdala inputs to the basal domain. Based on both animal models (Padival et al, 2013) and clinical studies (see the study by Price and Drevets (2010)) it has been suggested that there is disproportionate negative influence of the amygdala in mood disorders. We propose that ketamine, by restoring the strength of weakened apical inputs in all subregions of mPFC, would ameliorate this imbalance.
The ability of the mTORC1 blocker rapamycin to completely prevent ketamine enhancement of CRF-induced EPSCs is consistent with previous studies showing its synaptogenic and antidepressant effects of ketamine depend on activation of downstream targets of mTORC1 such as p70S6K (see the study by Duman and Aghajanian (2012)). It should be noted that ketamine activation of p70S6K also has been found in nucleus accumbens, hippocampus, as well as in BLA (Tedesco et al, 2013). It is possible that increased synaptogenesis in these as well as other regions also contribute to enhancements of connectivity throughout mood circuits. It remains to be determined if different aspects of antidepressant behavioral responses to ketamine overlap completely or only partially within the diverse regions where mTORC1 is activated.
BLA lesions led to a substantial reduction in CRF-induced EPSCs in layer V cells but 5-HT-induced EPSCs were unaffected by the lesions. In contrast, disconnection of the distal apical dendrite of mPFC layer V neurons did not significantly reduce in the CRF-induced EPSC responses but markedly decreased 5-HT-induced EPSCs, as expected from previous studies (Liu and Aghajanian, 2008). These results indicate that CRF-activated amygdala inputs are primarily targeted to the basal dendrites (see Supplementary Information and Supplementary Figure S2). In agreement with the electrophysiological data, there was a significant decrease in basal dendritic spine density of layer V neurons after BLA lesions. Nevertheless, more than half the spines remain after the lesions since the basal dendrites also receive non-BLA inputs, including a dense network of axon collaterals emanating from neighboring pyramidal cells (not shown).
In both IL and PL, chronic stress caused a decrease in apical/5-HT-induced EPSCs but no effect on CRF-induced synaptic responses. On the other hand, ketamine increases apical/5-HT-induced EPSCs in both PL and IL while CRF-induced EPSPs are increased in PL but are not altered in the IL. These electrophysiological results were paralleled by the effects of ketamine on spine density, which was increased in apical dendrites of both PL and IL but in basal dendrites the increase was seen only in PL. This dissociation between PL and IL suggests that there may be a compartmentalized suppression of activity-dependent synaptogenesis in the basal domain of IL neurons. This could occur via BLA innervation of a population of parvalbumin interneurons, which consist of basket and/or chandelier cells (Gabbott et al, 2006). The basket cells form a perisomatic distribution of terminals on basal dendrites of prefrontal layer V pyramidal neurons, which can exert compartmentalized inhibition of synaptic plasticity specific to the basal dendritic domain (Bar-Ilan et al, 2013). The possibility that the inhibitory component of the BLA input could selectively inhibit ketamine’s synaptogenic effect remains to be explored experimentally.
Numerous other anatomical and functional differences between IL and PL have also been reported, including differences in the pattern of their reciprocal connections with BLA (Gabbott et al, 2012; Marek et al, 2013). In behavioral studies, inactivation of IL does not alter fear expression but impairs acquisition of fear extinction and extinction memory. In contrast, inactivation of PL impairs fear expression but has no effect on fear memory (Sierra-Mercado et al, 2011). Distinct but intermingled subsets of BLA pyramidal cells project separately to PL and IL: the BLA cells targeted to PL show heightened activity during states of fear while the IL targeted cells are recruited during fear extinction (Senn et al 2014). IL has been implicated in resilience to fear-inducing social stress (Lehmann and Herkenham, 2011) while the opposite was found for PL (Vialou, et al, 2014). The projections of PL and IL subregions to the amygdala show distinct patterns of connectivity (Figure6). For example, there is a prominent projection of IL to the intercalated clusters of GABAergic neurons, which are situated between BLA and the central nucleus of the amygdala (CeA), enabling it exerts a strong inhibitory influence on fear responses by suppressing the amygdaloid output from the CeA (Royer et al, 1999; Berretta et al, 2005; Cho et al, 2013). Suppression of amygdala output may be of particular relevance to stress linked disorders such as anxiety and depression since chronic stress has been shown to cause an increase in excitatory drive of basal amygdala neurons, thereby causing disinhibition of CeA and widespread dissemination of stress signals (Padival et al, 2013). Thus, the differential effects of ketamine we see on CRF-induced responses in PL and IL could contribute to in the antidepressant actions of this drug in treatment-resistant patients.
Schematic diagram depicting the opposing roles of PL and IL on amygdala output. PL is seen as projecting to a subtype of BLA pyramidal cell that directly activates output cells of Ce, which project to regions such as brain stem and spinal cord that mediate stress responses. In contrast, IL is shown projecting to a different set of BLA pyramidal cells that activate inhibitory interneurons of ITC, which suppress Ce output (excitatory, green; inhibitory, red). BLA, basolateral amygdale; EPSC, excitatory postsynaptic current; IL, infralimbic; PL, prelimbic.
In conclusion, this study presents novel data on mTORC1-dependent synaptogenic effects of ketamine on CRF-activated amygdala inputs to basal dendrites of mPFC pyramidal cells. These studies extend previous work demonstrating mTORC1-dependent synaptogenic effects on input to apical dendrites (see the study by Duman and Aghajanian (2012)). Within the chronic stress model of depression, ketamine can be seen as restoring balance between atrophic apical inputs in relation to basal inputs, particularly in the IL subregion of mPFC, potentially overcoming the hypothesized negative influence of the amygdala in mood disorders. However, because of its acute psychotomimetic and possible longer term adverse effects, ketamine can be considered primarily a prototype drug that might lead to the development of agents that have antidepressant actions without acute psychosis. For example, recently it has been reported that the drug GLYX-13, an allosteric negative modulator rather than a direct blocker of the NMDA channel, has rapid and prolonged antidepressant actions without psychotomimetic effects in humans (Moskal et al, 2014) and in animal models induces an activation in mTORC1 signaling pathway that is associated with antidepressant-like action (Lu et al, 2014).
Funding and Disclosure
This work is supported by NIMH R37MH45481 (RSD), NIMH R01MH93897 (RSD), the State of Connecticut, and Yale University. The authors declare no conflict of interest.
References
Aghajanian GK, Marek GJ (1997). Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36: 589–599.
Bacon SJ, Headlam AJ, Gabbott PL, Smith AD (1996). Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res 720: 211–219.
Banasr M, Duman RS (2008). Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64: 863–870.
Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS (2007). Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry 62: 496–504.
Bar-Ilan L, Gidon A, Segev I (2013). The role of dendritic inhibition in shaping the plasticity of excitatory synapses. Front Neural Circuits 6: 118.
Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D (2005). Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132: 943–953.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354.
Cho JH, Deisseroth K, Bolshakov VY (2013). Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80: 1491–1507.
Cook SC, Wellman CL (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60: 236–248.
Duman RS, Aghajanian GK (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science 338: 68–72.
Gabbott PL, Warner TA, Busby SJ (2006). Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139: 1039–1048.
Gabbott P, Warner TA, Brown J, Salway P, Gabbott T, Busby S (2012). Amygdala afferents monosynaptically innervate corticospinal neurons in rat medial prefrontal cortex. J Comp Neurol 520: 2440–2458.
Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF (2010). Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J Neurosci 30: 16970–16982.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214.
Krystal JH, Sanacora G, Duman RS (2013). Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73: 1133–1141.
Lambe EK, Aghajanian GK (2003). Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron 40: 139–150.
Lehmann ML, Herkenham M (2011). Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci 31: 6159–6173.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69: 754–761.
Liu RJ, Aghajanian GK (2008). Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA 105: 359–364.
Little JP, Carter AG (2013). Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci 33: 15333–15342.
Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X et al (2014). PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice involve in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol 18: pyu110.
Magariños AM, McEwen BS (1995). Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69: 89–98.
Marek R, Strobel C, Bredy TW, Sah P (2013). The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 591: 2381–2391.
Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M (2013). Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol 16: 2181–2194.
Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF et al (2014). GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 23: 243–254.
Padival M, Quinette D, Rosenkranz JA (2013). Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology 38: 1748–1762.
Price JL, Drevets WC (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology 35: 192–216.
Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR et al (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125: 1–6.
Rostkowski AB, Leitermann RJ, Urban JH (2013). Differential activation of neuronal cell types in the basolateral amygdala by corticotropin releasing factor. Neuropeptides 47: 273–280.
Royer S, Martina M, Paré D (1999). An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 19: 10575–10583.
Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J et al (2014). Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81: 428–437.
Sierra-Mercado D, Padilla-Coreano N, Quirk GJ (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36: 529–538.
Tedesco V, Ravagnani C, Bertoglio D, Chiamulera C (2013). Acute ketamine-induced neuroplasticity: ribosomal protein S6 phosphorylation expression in drug addiction-related rat brain areas. Neuroreport 24: 388–393.
Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C et al (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212.
Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM et al (2014). Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci 34: 3878–3887.
Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP et al (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540.
Zarate C, Duman RS, Liu G, Sartori S, Quiroz J, Murck H (2013). New paradigms for treatment-resistant depression. Ann NY Acad Sci 1292: 21–31.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Liu, RJ., Ota, K., Dutheil, S. et al. Ketamine Strengthens CRF-Activated Amygdala Inputs to Basal Dendrites in mPFC Layer V Pyramidal Cells in the Prelimbic but not Infralimbic Subregion, A Key Suppressor of Stress Responses. Neuropsychopharmacol 40, 2066–2075 (2015). https://doi.org/10.1038/npp.2015.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.70
This article is cited by
-
Ethanol withdrawal-induced adaptations in prefrontal corticotropin releasing factor receptor 1-expressing neurons regulate anxiety and conditioned rewarding effects of ethanol
Molecular Psychiatry (2022)
-
Structure and function differences in the prelimbic cortex to basolateral amygdala circuit mediate trait vulnerability in a novel model of acute social defeat stress in male mice
Neuropsychopharmacology (2022)
-
Loss of liver X receptor β in astrocytes leads to anxiety-like behaviors via regulating synaptic transmission in the medial prefrontal cortex in mice
Molecular Psychiatry (2021)
-
Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions
Molecular Psychiatry (2020)
-
N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects
Neuropsychopharmacology (2019)