Abstract
Despite the strong association between impulsivity and addiction in humans, it is still a matter of debate whether impulsive choice predisposes to, or results from, drug dependence. Furthermore, it is unknown whether treating impulsivity can protect against relapse propensity. Therefore, this study explored the bidirectional relationship between impulsive choice and cocaine taking and seeking in rat behavioral models. In experiment 1, to determine whether impulsive choice predisposes to cocaine taking or seeking, rats were selected based on trait impulsivity in a delayed reward task and subsequently compared on various stages of cocaine self-administration (SA). To examine the consequence of cocaine intake on impulsive choice, impulsivity was monitored once a week throughout various stages of cocaine SA. To determine whether treating impulsive choice can protect against relapse propensity, in experiment 2, impulsive choice was manipulated by pharmacological interventions and cocaine-associated contextual cues. Trait impulsive choice as determined in experiment 1 predicted high extinction resistance and enhanced propensity to context-induced relapse in the cocaine SA model, whereas cocaine intake did not alter impulsive choice. Furthermore, acute changes in impulsive choice were not related to rates of context-induced relapse. Taken together, the current data indicate that trait impulsive choice predicts persistent cocaine seeking during extinction and enhanced propensity to relapse, whereas acute manipulations of impulsive choice had no favorable outcomes on relapse measures. These observations suggest that trait impulsivity can be used as a predictive factor for addiction liability, but treating this impulsivity does not necessarily protect against relapse.
Similar content being viewed by others
INTRODUCTION
There is a broad consensus that drug dependence and impulsivity are closely related, although whether impulsivity precedes or results from chronic drug use is difficult to dissect from existing clinical data (De Wit, 2009; Perry and Carroll, 2008; Setlow et al, 2009; Winstanley et al, 2010). An important implication of the hypothesis that impulsivity predisposes to drug dependence (Jentsch and Taylor, 1999; Winstanley et al, 2010) is that pharmacotherapies ameliorating impulsivity might be a promising treatment strategy in drug dependence. In this study, we aim to (1) gain further insights into the interrelationship between cocaine seeking and trait impulsivity and (2) explore whether manipulation of impulsivity through pharmacological treatment can affect relapse to cocaine seeking.
Impulsivity, although widely viewed as action without foresight, is multifaceted in nature and consists of distinct behavioral modalities. Thus, impulsive behavior might arise from deficient inhibitory response control (impulsive action) and/or from difficulties evaluating long-term behavioral consequences and delay aversion (impulsive choice) (for reviews, see Evenden (1999) and Winstanley et al (2006)). Prospective human studies have revealed that deficient inhibitory response control during childhood predicts alcohol and illicit drug use later in life (Nigg et al, 2006; Tarter et al, 2003; Wong et al, 2006), and that trait impulsivity predicts stimulant abuse (Ersche et al, 2010) and treatment outcome in a smoking cessation program (Krishnan-Sarin et al, 2007). Interestingly, preclinical animal studies have indicated that pre-existing levels of impulsive action mainly predict volitional cocaine (Belin et al, 2008; Dalley et al, 2007) and nicotine consumption (Diergaarde et al, 2008), whereas impulsive choice may predict persistent drug seeking during extinction and the enhanced vulnerability to reinstate drug seeking (Diergaarde et al, 2008; Perry et al, 2008). From a clinical perspective, treatment interventions that maintain abstinence and prevent relapse are most relevant; therefore, this study focused on trait impulsive choice.
Until now, most preclinical studies on the relationship between impulsivity and drug addiction have either focused on drug self-administration (SA) after establishment of baseline impulsivity levels or on impulsivity levels after drug administration (for reviews, see Perry and Carroll (2008), Setlow et al (2009), and Winstanley et al (2010)). Only a few studies have determined impulsivity levels at various stages of volitional drug SA (Dalley et al, 2005a, 2005b; Diergaarde et al, 2008; Gipson and Bardo, 2009; Winstanley et al, 2009). Taken together, these studies revealed that trait impulsivity may predict drug taking and/or seeking and that (passive) drug intake can (transiently) increase impulsive behavior. Thus far, only a single study has monitored impulsive action levels throughout cocaine-taking and cocaine-seeking episodes (Winstanley et al, 2009), and whether impulsive choice is affected during episodes of cocaine SA has not been examined before. To address whether impulsive choice represents a risk factor for cocaine taking or seeking, we characterized rats as high or low in trait impulsive choice using a delayed reward paradigm and subsequently subjected these individuals to volitional cocaine SA, extinction, and reinstatement. To assess whether impulsive choice changed during cocaine SA, impulsivity levels were continuously monitored throughout the course of cocaine taking and seeking.
From a clinical and therapeutic perspective, the critical question arises as to whether elevated impulsivity can be reduced to promote and support abstinence and reduce the propensity to relapse in drug-dependent individuals. In this respect, previous studies have demonstrated beneficial effects of the clinically used norepinephrine transporter inhibitor atomoxetine on impulsivity (Blondeau and Dellu-Hagedorn, 2007; Chamberlain et al, 2007; Paterson et al, 2011; Robinson et al, 2008) and drug taking or seeking (Economidou et al, 2011; Wilens et al, 2008), supporting this notion. Nonetheless, it remains unclear whether the observed attenuation in drug seeking is actually related to the reduction in impulsivity. This study further explored this interrelationship by pharmacologically manipulating both impulsive choice and context-induced reinstatement. First, impulsive choice was either reduced with the clinically relevant drug methylphenidate or increased with the dopamine D1 receptor antagonist SCH-23390 as shown previously (eg, van Gaalen et al, 2006b), and subsequently, these drug effects were assessed on context-induced reinstatement within the same individuals. Second, as drug-associated contextual stimuli are very powerful in reinstating drug seeking, we determined whether exposure to cocaine-associated contextual stimuli would affect impulsive choice. Collectively, these manipulations might provide further insights into the directionality of the relationship between impulsive-choice cocaine-seeking behavior.
MATERIALS AND METHODS
General Procedures
Animals
Male Wistar rats (Harlan, Horst, The Netherlands), initially weighing 240–270 g, were housed in standard Macrolon cages on a reversed 12-h day/night cycle (lights on at 1900 hours) in a temperature (21±2°C)- and humidity (50±10%)-controlled room. Rats were housed in pairs until surgery, and individually afterwards. Behavioral testing was conducted during the dark phase of the day/night cycle. During the entire experiment, rats were food restricted, and maintained at about 85–90% of their free-feeding weight, by providing them with 14–18 g of chow at the end of each day. Water was available ad libitum. Experiments were approved by the Animal Care Committee of the Free University of Amsterdam (The Netherlands).
Delayed reward task
Detailed descriptions of apparatus and training procedures were provided previously (van Gaalen et al, 2006b) and in Supplementary Methods. In brief, during the final stages of training, a session was divided into 5 blocks of 12 trials; each block started with 2 forced choice trials. Each rat received a left forced and a right forced trial. The order of these was counterbalanced between subjects. In the next 10 trials, animals had a free choice and both the left and right units were illuminated. Poking into one position resulted in immediate delivery of a small reinforcer (one food pellet), whereas a nose poke into the other position resulted in delivery of a large, but delayed, reinforcer (four food pellets). If an animal did not make a response during this choice phase within 10 s, an intertrial interval was initiated and the trial was counted as an omission. The position associated with the small and large reinforcers was always the same for each individual, and counterbalanced for the group of rats. Delays for the large reinforcer progressively increased within a session per block of 12 trials as follows: 0, 5, 10, 20 and 40 s. Responding into non-illuminated units during the test was recorded, but had no further programmed consequences. The behavioral measure to assess task performance, ie, the percentage preference for the large reinforcer as a function of delay, was calculated as the number of choices for the large reinforcer/(number choices large+small reinforcers) × 100. Furthermore, we calculated the number of omitted choice trials per block of 10 trials within a session and the indifference point. This indifference point, defines the delay at which animals have a 50% preference for the large reward and was based on the equation proposed by Mazur (1987), where preference big reward=preference at delay 0/(1+k × delay), and parameter k represents the steepness of the discounting curve.
Cocaine SA
Rats were prepared for i.v. catheter surgery by providing ad libitum access to food for at least 3 days before surgery. Intravenous silicon catheters (outer diameter: 0.78 mm, inner diameter: 0.5 mm) were surgically implanted in the right jugular vein under gas anesthesia (isoflurane). The catheter was secured to the vein with two sutures and passed subcutaneously to the top of the skull. The distal end of the catheter was attached to a connector pedestal (Plastics One, Dusseldorf, Germany) anchored to the skull using four surgical screws and dental cement. Rats received 0.5 ml/kg of the analgesic Ketofen (1%; Merial) and 0.3 ml/kg of the antibiotic Baytril (2.5%; Bayer) at the end of surgery. Catheter patency was maintained by daily infusion of 0.1 ml of sterile saline solution containing heparin (47.5 IU/ml) and gentamicin (0.08 mg/ml). After surgery, animals were housed individually and had ad libitum access to food for at least 5 days before retraining in the DRT.
Training of rats occurred in 32 rat two-lever operant chambers (Med Associates, St Albans, VT, USA). All chambers were equipped with a red house light, a white noise generator (ENV-225SM, Med Associates), and a liquid swivel connecting rats to an infusion pump (PHM-100, Med Associates, total volume of 42.52 μl delivered over 2 s). Rats were randomly assigned to one of two different contexts used for either cocaine or saline SA. These contexts differed in (1) white noise (70 dB), either continuous or interval (5 s on, 5 s off), (2) odor, either lemon-scented or almond-scented, and (3) the chamber floor, a flat PVC surface with either holes or straight grooves. The cocaine-taking and cocaine-seeking paradigm included the following stages:
SA, FR1: Acquisition of cocaine (Cocaine-HCl, OPG, Utrecht, The Netherlands) and saline responding occurred according to a fixed ratio 1 schedule of reinforcement. In daily 3-h sessions, active lever presses resulted in an infusion of sterile saline or cocaine. Rats received 250 μg/kg per infusion of cocaine during the first 5 sessions, followed by 500 μg/kg per infusion for the remaining sessions. To prevent overdosing in inexperienced rats, the maximum number of rewards was set at 40 for the first three and at 100 for the next two sessions. After this, animals could earn an unlimited number of infusions. There was a non-signaled time out of 15 s after every infusion. Inactive lever presses were registered, but without consequences.
Dose-response curve: On three consecutive days, rats received two 1-h sessions per day (with 1 h in between) on a fixed ratio 1 schedule (adapted from Dalley et al (2007)). The sensitivity to respond for cocaine was determined over these 6 sessions, in a descending dose order in all rats with doses of 500, 250, 125, 62.5, 31.25 and 15.625 μg/kg cocaine. Sterile saline infusions were unchanged for all sessions.
Progressive ratio: The motivation to self-administer cocaine was determined in three identical sessions on three consecutive days (only the last two days were used for analysis). Sessions ended after 4 h or after 1 h without earning a reward, whichever occurred first. The number of active responses leading to one infusion was increased along the following equation: response ratio=5 × e(0.2 × infusion number)−5, rounded to the nearest integer.
Extinction: Extinction training consisted of daily, 1-h sessions in a neutral context. The house light was illuminated, but there was no odor induced, white noise was absent, and there was a grid floor. Active and inactive responses were registered, but without consequences.
Context-induced reinstatement: Context-induced reinstatement was performed by reintroduction of the cocaine- or saline-associated context into the operant chamber (white noise, odor, and PVC floor). During the 30-min session, active and inactive responses were registered, but without consequences.
Context extinction: Conditions were similar to those used during the context-induced reinstatement test, with the only exception that sessions lasted 1 h per day. In this phase, differences in extinction responding in the cocaine context are determined and this precedes cocaine- and stress-induced reinstatement tests. Previous work has indicated that cocaine- and stress-induced reinstatement is more robust in the presence of previously extinguished drug-associated cues. All lever responses were registered, but without consequences.
Cocaine-induced reinstatement: Cocaine-induced reinstatement was performed under context extinction conditions, but lasted 30 min per session. Twenty minutes before the test, all rats received intraperitoneal injections of saline (1 ml/kg) on the first test day and cocaine (10 mg/kg) on the next test day.
Stress-induced reinstatement: After five additional context extinction sessions, a stress-induced reinstatement test was performed. In a 30-min session, under context extinction conditions, stress was induced with the α2-adrenoceptor antagonist yohimbine (1.25 mg/kg, yohimbine-HCl, Sigma, St Louis, MI), which has previously been shown to provoke similar effects as foot shock-induced stress (Shepard et al, 2004). Rats were first injected with sterile water (1 ml/kg) and the next day with yohimbine (1.25 mg/kg) 45 min before testing.
Experiment 1: Risk Factor or Consequence
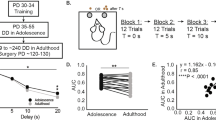
Experiment 1 was performed to determine whether impulsive choice predisposes or results from cocaine taking and seeking. For this purpose, 96 rats were trained in the DRT (Figure 1a). Upon stable baseline performance, upper (high impulsive, HI) and lower (low impulsive, LI) quartiles were selected based on their indifference points and equipped with jugular catheters. After re-establishment of stable DRT performance, rats were subjected to all of the above-mentioned behavioral stages of drug taking and seeking. Half of the rats were trained to self-administer cocaine (14 HI and 14 LI) and the other half saline (12 HI and 12 LI) according to the following schedule: SA on FR1 schedule (13 sessions), dose-response curve, progressive ratio, extinction (16 sessions), context-induced reinstatement, context extinction (14 sessions), cocaine- and stress-induced reinstatement. To determine the consequence of cocaine intake on impulsivity, rats were tested once weekly in the DRT during the entire course of cocaine or saline taking and seeking. Two LI animals did not complete the experiment (one because of catheter failure and one because of disturbed DRT behavior after surgery), and were therefore excluded from all analyses.
Schematic diagram depicting the experimental design. Experimental design of experiments 1 (a) and 2 (b). Fill colors identify the correspondence in contexts. DRT, delayed reward task; HI, high impulsive; LI, low impulsive; SA, self-administration.
Experiment 2: Treatment Potential
This experiment was designed to explore the treatment potential of impulsivity as a risk factor for relapse. To that end, we compared manipulations of impulsive choice and context-induced reinstatement in the same animals using a counterbalanced within subjects design. As this experiment involved correlation analyses, no pre-selection on high and low impulsivity was performed. A new cohort of 32 rats was trained in the DRT (Figure 1b) and upon stable baseline performance equipped with jugular catheters. Upon re-establishment of stable DRT performance, all rats were trained to self-administer cocaine and saline in different contexts. To strengthen differentiation between the cocaine and saline context, saline SA started after rats showed a clear preference for the active lever on cocaine SA (seven sessions). Rats were then trained to self-administer 0.9% physiological saline in the mornings, along with cocaine in the afternoon (12 days). Upon stable drug intake, the effect of the cocaine- and the saline-associated context on impulsive choice was measured (DRT context test). In this test, the cocaine and saline contextual cues (white noise, odor and grid floor) were introduced into the DRT operant chamber. Each animal was tested under the cocaine and saline context in a counterbalanced design. After this, all rats were subjected to a 15-day extinction period (2 sessions per day) in a neutral context, followed by another DRT context test. Hereafter, context-induced reinstatement tests were performed 10 min after subcutaneous injections of either saline or SCH-23390 (R(+)-SCH-23390 HCl, Sigma, 10 μg/kg) in the cocaine SA context in a counterbalanced, within-subjects manner. In between reinstatement tests lapsed 1 week of further extinction training. During the next 3 weeks, the effects of SCH-23390 and the monoamine transporter inhibitor methylphenidate (0.5 and 1.0 mg/kg intraperitoneal) on impulsive choice were tested. This was followed by the final context-induced reinstatement tests, 10 min after intraperitoneal injections of saline or methylphenidate (1 mg/kg) in the cocaine SA context, performed in a counterbalanced, within-subjects manner. In between both context-induced reinstatement tests lapsed 1 week of context extinction. During the entire cocaine SA paradigm, rats were trained in the DRT once weekly. In total, eight animals did not complete the experiment (two because of instable DRT performance, four because of catheter failure, and two because of disturbed DRT behavior following the cocaine SA protocol) and were therefore excluded from all analyses.
Statistical Analyses
Data were analyzed using repeated-measures analysis of variance (ANOVA) and were adjusted for violation of homogeneity of variances using Huyn–Feldt ɛ. For SA data, sessions and lever (active vs inactive) served as within-subjects variables. The SA drug (saline or cocaine) served as between-subjects factor in experiment 1 and as within-subjects variable in experiment 2. Moreover, in experiment 1, the pre-existing level of impulsivity was an additional between-subjects factor. Dependent variables were the number of active and inactive responses. For the DRT data, dependent variables were preference for the large reward, and number of omitted choice trials, and within-subjects variables were delay to the large reward and drug treatment. Only for experiment 1, the between-subjects factor impulsivity (high vs low) was included. Pearson's correlation analyses were used to test whether reactivity to the SA-associated context and drug challenges in the DRT (SCH-23390 and methylphenidate) related to reactivity in reinstatement tests. Data were analyzed using the Statistical Package for the Social Sciences version 15.0 (SPSS, Chicago, IL, USA), and the significance level was set at p<0.05.
RESULTS
Experiment 1: Trait Impulsivity and Cocaine Seeking
Impulsive choice throughout distinct stages of cocaine taking and seeking
Selection of HI and LI animals was based on indifference points and occurred following stable baseline levels of impulsive choice, which took 28 sessions in the final training stage. The pre-existing indifference point of HI rats (12.6±1.7 s) was lower than that of LI rats (44.9±3.9 s) (impulsivity: F(1, 49)=59.2, p<0.001), with no difference between cocaine and saline SA groups. Moreover, groups did not differ in terms of the number of omissions (HI: 2.0±0.6 and LI: 1.9±0.4 per block of 10 trials) during the choice phase.
During the entire SA procedure, indifference points remained constant (Figure 2a). HI rats had lower indifference points than did LI rats (impulsivity: F(1, 34)=17.18, p<0.001) with no difference between cocaine and saline SA subgroups. Stable levels of impulsive choice (0.90) and omissions (0.79) were confirmed by high test–retest reliability (Cronbach's α) over all 10 sessions. Further in-depth analysis of cocaine SA animals revealed that indifference points of LI animals taking on average >30 mg/kg per session (n=8), decreased (reflecting an increase in impulsivity) during SA, which returned to baseline during extinction (session: F(11, 77)=6.13, p<0.001)). These animals also omitted fewer trials during cocaine SA and extinction (omissions: F(1, 10)=9.71, p<0.05). Conversely, both indifference points and omissions of HI animals remained stable during the course of the experiment. In all animals, HI and LI, impulsive choice levels at the end of the experiment were not different from the pre-existing levels.
Impulsive choice during distinct phases of cocaine taking and seeking. Indifference points remained stable over weekly DRT sessions (a), with a high test–retest reliability (Cronbach's α: 0.9). High-impulsive (HI) animals remained more impulsive than low-impulsive (LI) animals and there was no overall group difference between cocaine and saline SA. **p<0.001 LI vs HI animals. Within the cocaine SA animals (b), depending on the intake impulsive choice transiently changed in subgroups of rats and in particular LI animals with high intake (on average >30 mg/kg per session) became more impulsive compared with LI low-intake animals (intake, within LI group: #p<0.05).
Trait impulsive choice predicts cocaine seeking
As the saline SA control group did not show a preference for the active lever, and inactive responding remained constant over the entire experiment in both groups, these results were not presented. Over the first five acquisition sessions, active lever responding for cocaine increased (Supplementary Figure 3a, session: F(5, 125)=17.89, p<0.001, ɛ=0.4), but there was no difference between HI and LI rats. After acquisition, cocaine intake was stable over the eight unlimited cocaine-taking sessions and equal for LI and HI rats. The dose-response curve for cocaine followed an inverted U shape (session: F(5, 125)=28.68, p<0.001, ɛ=0.7), but there was no difference between HI and LI rats (Supplementary Figure 3b). During the progressive ratio sessions, HI (1206±235) and LI rats (827±208) made a comparable amount of active lever responses for cocaine (Supplementary Figure 3c).
HI rats showed a stronger resistance to extinction of cocaine seeking than did LI rats (Figure 3a, session*impulsivity: F(15, 375)=3.63, p<0.05, ɛ=0.3). Nonetheless, both in HI (F(15, 195)=9.18, p<0.001) and in LI (F(15, 180)=3.67, p<0.001), animals active lever responding decreased over the course of extinction sessions. After extinction, re-exposure to the cocaine-associated context induced an increase in active responding in both HI (F(1, 11)=45.56, p<0.001) and LI (F(1, 10)=26.41, p<0.001) animals. Nonetheless, the context-induced reinstatement response was stronger in HI than in LI rats (see Figure 3b, session*impulsivity: F(1, 21)=9.59, p<0.05). The difference between HI and LI animals was evident within the first 5 min of the reinstatement test (impulsivity: F(1, 22)=7.22, p<0.05), implying that this effect is not caused by differences in extinction resistance, but by higher cue reactivity in the HI group. Similar to the extinction phase described earlier, during context-extinction sessions, active lever responding extinguished (Supplementary Figure 3d, session: F(13, 325)=34.06, p<0.001), with HI animals showing more active lever responses than LI animals (impulsivity: F(1, 25)=5.21, p<0.05). HI and LI rats showed a comparable drug-induced reinstatement response to the cocaine prime (10 mg/kg), compared with the saline injection (Figure 3c, session: F(1, 25)=37.85, p<0.001). In addition, the stress-induced reinstatement response to the pharmacological challenge with the preferential α2-adrenoceptor antagonist yohimbine (1.25 mg/kg) was also similar for HI and LI rats (Figure 3d, session: F(1, 25)=10.50, p<0.05).
Extinction (a) and reinstatement (b–d) of cocaine seeking in experiment 1. During extinction (panel a), high-impulsive (HI) rats were more resistant to extinction than were low-impulsive (LI) rats (session*impulsivity: #p<0.05). However, in both HI and LI animals, active lever responding decreased over sessions (session: **p<0.001). Both HI and LI animals showed a context-induced reinstatement response (session: **p<0.001) and this was stronger in HI than in LI animals (panel b, session*impulsivity: #p<0.05). Both cocaine (panel c, 10 mg/kg) and yohimbine (panel d, 1.25 mg/kg) induced reinstatement of cocaine seeking, but these effects did not differ between HI and LI animals (session: *p<0.05, **p<0.001).
Experiment 2: Transient Changes in Impulsivity and Cocaine Seeking
Cocaine SA paradigm
During the first seven cocaine SA sessions, rats developed a preference for the active lever (session*lever: F(6, 72)=4.64, p<0.05, ɛ=0.6). The following 12 days, rats self-administered both cocaine and saline and showed a preference for cocaine (Supplementary Figure 4a, session*drug: F(11, 187)=7.61, p<0.001). During the extinction phase, both the ratio of active-to-inactive lever responses (session*lever: F(14, 350)=5.59, p<0.001, ɛ=0.6), and responding for cocaine to saline (session*drug: F(14, 350)=4.03, p<0.001, ɛ=0.6) decreased within 16 sessions (Supplementary Figure 4b). After extinction, cocaine seeking was reinstated by the cocaine-associated context (session: F(1, 23)=43.40, p<0.001). Furthermore, as shown in Figure 4, reinstatement of cocaine seeking was attenuated by the preferential dopamine D1 receptor antagonist SCH-23390 (treatment: F(1, 23)=23.59, p<0.001), whereas the monoamine transporter inhibitor methylphenidate potentiated reinstatement of cocaine-seeking behavior (treatment: F(1, 23)=27.33, p<0.001).
Reinstatement of cocaine seeking. The context-induced reinstatement of cocaine seeking was blocked by the preferential dopamine D1 receptor antagonist SCH-23390 (10 μg/kg) and potentiated by the monoamine transporter inhibitor and stimulant methylphenidate (1 mg/kg). *p<0.05 and **p<0.001 compared with extinction (ext); #p<0.05 compared with saline injection.
Impulsive choice throughout cocaine SA and extinction
During the course of cocaine SA and extinction, both impulsive choice (indifference point) and omissions remained stable (Cronbach's α indifference points: 0.80, omissions: 0.81). Pharmacological challenges with SCH-23390 increased impulsive choice (Figure 5a, treatment: F(4, 84)=5.63, p<0.05, ɛ=0.7) and slightly increased the number of omissions (see Supplementary Figure 2, omissions: F(1, 23)=23.04, p<0.05). In contrast to the effects of SCH-23390, impulsive choice was decreased by both doses of methylphenidate (Figure 5b, delay*treatment: F(8, 148)=2.80, p<0.05). Moreover, methylphenidate decreased the number of omissions (treatment: F(2, 46)=3.41, p<0.05) and this occurred only at the low dose.
Transient manipulations of impulsive choice. Significant changes in impulsive choice were observed after acute challenges with SCH-23390 (a), methylphenidate (b), or exposure to the cocaine-associated context (c). *p<0.05 compared with saline injections (panels a and b) or saline context (panel c).
In the DRT, exposure to the cocaine-associated context strongly enhanced the number of omissions in a subgroup of n=7 animals (in comparison with the saline-associated context). As no reliable delay discounting curve could be determined in these animals, they were excluded from further analyses. The remaining 17 animals displayed equal amounts of omissions in both contexts (cocaine: 15±2.8%, saline 16±0.3%). In these 17 animals, impulsive choice was decreased in the cocaine context than in the saline context (Figure 5c, context: F(4, 60)=3.40, p<0.05). These effects of cocaine-associated contextual cues on impulsive choice and omissions were observed on the first days of abstinence, but not in a second test, which was performed after 3 weeks of extinction training.
Correlation analyses of transient changes in impulsivity as predictors of context-induced reinstatement of cocaine seeking
As described above, the cocaine-associated context induced reinstatement of cocaine seeking and at the same time decreased impulsive choice in the DRT. Importantly, these effects on cocaine seeking and impulsive choice were not correlated (R=0.30, NS). Similarly, although acute drug challenges with methylphenidate decreased impulsive choice, this same manipulation potentiated context-induced reinstatement. Notably, these drug effects were also not correlated (R=0.22, NS). Opposite to the effects of methylphenidate, drug challenges with SCH-23390 increased impulsive choice and decreased context-induced reinstatement with no correlation between these drug effects (R=0.15, NS).
DISCUSSION
This study examined the direction of the relationship between impulsive choice and the propensity to take and seek cocaine. These experiments revealed that trait levels of impulsive choice predict both resistance to extinction and vulnerability to context-induced relapse to cocaine seeking. In contrast, cocaine intake patterns, including the sensitivity and motivation to self-administer cocaine, as well as drug- or ‘stress’-induced relapse were not related to trait impulsive choice. Vice versa, daily SA of cocaine marginally and transiently increased impulsive choice under drug-free conditions, whereas exposure to the cocaine-associated context cues surprisingly reduced impulsive choice. Interestingly, pharmacological manipulation of impulsive choice by the dopamine D1 receptor antagonist SCH-23390 or the monoamine transporter inhibitor methylphenidate did not predict corresponding changes in the propensity to context-induced relapse.
Trait Impulsive Choice Predicts Cocaine Seeking
The pre-existing level of impulsive choice predicted resistance to extinction of cocaine seeking. Thus, compared with LI animals, HI rats displayed prolonged cocaine seeking both during extinction in a neutral context and in the cocaine-associated context. When responding was fully extinguished in both groups, context-induced relapse to cocaine seeking was much more pronounced in HI animals. These observations are strikingly similar to those obtained in HI and LI animals after nicotine SA (Diergaarde et al, 2008). In that study, nicotine cues consisted of light-tone combinations directly paired with drug infusion (discrete cues). In this study, we show that enhanced propensity to relapse in HI animals is observed after re-exposure to the cocaine-associated context. Therefore, high levels of impulsive choice relate to an enhanced sensitivity to stimulant-paired discrete and contextual cues to drive relapse. Nonetheless, in contrast to these observations, recent work indicates that impulsive choice does not predict different aspects of heroin taking and seeking (Schippers et al, 2011). Thus, these discrepant findings suggest that, impulsive choice does not predict the sensitivity to reward-related cues in general, but rather selectively predicts sensitivity to stimulant-related drug-paired cues.
Trait impulsive choice did not predict the sensitivity to ‘stress’-induced reinstatement of cocaine seeking as assessed upon a drug challenge with the preferential α2-adrenoceptor antagonist yohimbine (Shepard et al, 2004). Although it should be noted that only a single dose of yohimbine was used, the differential effects on context- vs stress-induced reinstatement observed here are consistent with the idea that the neurocircuitry mediating these processes (partially) differ. Thus, although there appears to be a final common pathway involved in relapse behavior (prefrontal accumbens), stress-induced, unlike context-induced cocaine seeking, depends on the integrity of the bed nucleus of stria terminalis. On the other hand, the hippocampus and basolateral amygdala have a more important role in context-induced reinstatement (for reviews, see Bossert et al (2005) and Shaham et al (2003)).
Reinstatement induced by a cocaine-priming injection was not different between HI and LI animals. Although we only tested a single dose, these data are in agreement with the study by Perry et al (2008), showing that HI female, but not male rats, displayed more robust cocaine-induced reinstatement (although only for the highest, 15 mg/kg, dose of cocaine). Taken together, these data indicate that the sensitivity to cocaine-induced relapse is not influenced by premorbid impulsivity levels, but that gender differences are an important consideration in this assessment. It is possible that, similar to stress-induced reinstatement, the observed differential effects of context- and drug-induced reinstatement in HI rats are explained by the underlying neural circuits. The prefrontal accumbens pathway is considered as the final common pathway involved in all forms of reinstatement, but the ventral pallidum, seems to be specifically involved in drug-induced reinstatement (for reviews, see Bossert et al (2005) and Shaham et al (2003)).
This divergence in neurocircuitry might help to explain the specific relationship between impulsive choice and context-induced reinstatement, as both of these behaviors are, at least in part, dependent on the integrity of the hippocampus (Cheung and Cardinal, 2005; Fuchs et al, 2005) and the basolateral amygdala (Park et al, 2002; Winstanley et al, 2004). Moreover, the basolateral amygdala also has an important role in regulating extinction of cocaine seeking (Fuchs et al, 2006), on which HI and LI animals also differed in this study and previously regarding nicotine seeking (Diergaarde et al, 2008).
Trait Impulsive Choice does not Predict Measures of Cocaine Taking
Trait impulsive choice did not predict cocaine intake, the sensitivity to cocaine, or the motivation to work for cocaine under a progressive ratio schedule. This is largely in line with previous observations in male and female rats, demonstrating no profound predictive potential of impulsive choice on cocaine intake during continuous and progressive ratio reinforcement schedules (Anker et al, 2009; Perry et al, 2008).
Interestingly, some earlier studies have shown that a different form of impulsivity, that is impulsive action as measured with the five-choice serial reaction time task, seemed much more predictive of cocaine and nicotine drug intake patterns and the motivation to self-administer these drugs (Belin et al, 2008; Dalley et al, 2007; Diergaarde et al, 2008). Taken together, this may indicate that impulsive action is mainly predictive of drug intake, escalation, motivation, and sensitivity to drugs, whereas drug seeking under extinction conditions, including relapse, may be more related to impulsive choice. In support of this and as outlined before, there is considerable neuroanatomical, neurochemical, and pharmacological evidence that impulsivity is not unitary (for reviews, see Evenden (1999), Pattij and Vanderschuren (2008), and Winstanley et al (2006)). However, this distinction does not seem unambiguous. For instance, it was recently shown that impulsive action was associated with certain aspects of cocaine seeking, such as the resistance to punishment after prolonged cocaine SA (Belin et al, 2008), as well as increased sensitivity to reinstate cocaine-seeking under punishment (Economidou et al, 2009). Further research is required to elucidate the commonalities and relationships between impulsive action and choice.
Effect of Cocaine History on Impulsive Choice
During the course of the entire cocaine SA protocol, rats were trained in the DRT once a week, thereby allowing the assessment of shifts in baseline impulsive choice. When averaged across all animals, impulsive choice did not shift at any point during or after different phases of cocaine SA. Furthermore, an acute challenge with cocaine in this study (see Supplementary Material and Supplementary Figure 1) reduced impulsive choice in the DRT in HI and LI animals similarly, and this was not different in saline or cocaine SA rats. Finally, there was no difference in the effect of SCH-23390 on impulsive choice before and after cocaine SA (see Supplementary Material). Collectively, these findings imply that the frequently observed elevated levels of impulsive behavior in cocaine-dependent individuals (Coffey et al, 2003; Kirby and Petry, 2004; Verdejo-Garcia and Perez-Garcia, 2007) are a pre-existing personality trait, and not merely a resultant from prolonged cocaine use. This observation is confirmed by a steeper discounting rate in both former and current cocaine users, in comparison with healthy controls (Heil et al, 2006).
In contrast to volitional cocaine SA in this study, forced cocaine administration has been reported to increase impulsive choice in laboratory animals (Paine et al, 2003; Roesch et al, 2007; Simon et al, 2007). Nevertheless, in this study, only a small subset of individuals, that is, LI animals with high cocaine intake (>30 mg/kg cocaine per session), temporarily became more impulsive, and this shift in impulsive choice was particularly evident during the cocaine SA phase under continuous reinforcement. In HI animals, this change in impulsive choice was not observed, possibly due to a floor effect of the indifference point. Thus, the former finding suggests that volitional cocaine intake is associated with changes in impulsive behavior in LI animals, but only when drug intake is high. This is supported by recent work demonstrating that rats trained to self-administer 30 mg/kg cocaine per day for 2 weeks indeed were more impulsive in a comparable DRT when tested 3 weeks after withdrawal (Mendez et al, 2010). The contrasting results between forced administration and our volitional cocaine SA approach could perhaps be attributed to differential neuroadaptations in these different administration methods (Jacobs et al, 2003; Winstanley et al, 2007).
Effects of Cocaine Context on Impulsive Choice
We observed that exposure to the cocaine-associated context, and not the neutral, saline-paired context, reduced impulsive choice. As cocaine by itself reduces impulsivity in this task (this study; Winstanley et al, 2007), it is possible that rats anticipated cocaine and showed an acute cocaine-like response to the context. This reduction of impulsive choice, induced by the cocaine context, was only observed in the first test immediately after the last cocaine SA session, and not in a re-test after long term (3 weeks) abstinence. The transient nature of this effect is puzzling and warrants further investigation. This observation is particularly interesting in view of observations that the propensity of cocaine-associated cues to provoke relapse increases over time, a phenomenon referred to as incubation of craving (Grimm et al, 2001, 2002). This may not hold true for the effects of these cues on impulsivity, suggesting that different neurobiological substrates are involved in these processes.
Pharmacologically Induced Changes in Impulsive Choice and its Repercussions for Cocaine Relapse
To further examine the bidirectionality between impulsive choice and the propensity to reinstate cocaine-seeking behavior, and to investigate the treatment potential of relapse through a reduction of impulsivity, we directly compared the effects of different drugs known to modulate both phenomena in a within-subjects approach. Acute challenges with the clinically relevant drug, methylphenidate clearly decreased impulsive choice in the DRT. However, in the same individuals, methylphenidate robustly potentiated context-induced reinstatement of cocaine seeking. This latter observation is consistent with previous work demonstrating that methylphenidate reinstates cocaine-seeking behavior in a SA paradigm (Schenk and Partridge, 1999). In contrast to methylphenidate, the preferential dopamine D1 receptor antagonist SCH-23390 was found to increase impulsive choice, in line with earlier data (van Gaalen et al, 2006b; Zeeb et al, 2010). In the same individuals, SCH-23390 attenuated context-induced cocaine seeking contrasting its effects on impulsive choice. Further correlation analyses revealed that these pharmacologically induced behavioral changes in each paradigm did not correlate. Interestingly, the effects of methylphenidate (Navarra et al, 2008), SCH-23390 (van Gaalen et al, 2006a), the serotonin2A receptor antagonist M100907 (Winstanley et al, 2003), and the clinically prescribed noradrenaline reuptake inhibitor atomoxetine (Robinson et al, 2008) on impulsive action do match the directionality of their effects on reinstatement of cocaine seeking (this study; Economidou et al, 2011; Nic Dhonnchadha et al, 2009). Therefore, it would be interesting to determine to what extent these drug effects on impulsive action and reinstatement to cocaine seeking would correlate in a similar approach adopted here. The current findings suggest that these behavioral phenomena are under differential neurobiological control, inasmuch as studied here with SCH-23390 and methylphenidate. Notably, in this study, only acute challenges with these drugs were investigated. Thus, in follow-up studies, it would be important to verify whether more prolonged administration of these drugs would affect the directionality of the effects. Nevertheless, our findings do imply that cocaine-dependent individuals may not benefit from methylphenidate to maintain abstinence and prevent relapse. This notion is supported clinically, as methylphenidate does improve measures of inhibitory control in cocaine-dependent individuals (Goldstein and Volkow, 2011; Li et al, 2010), despite the observation that the clinical effects of methylphenidate on treatment retention in cocaine dependence are less pronounced (Castells et al, 2010).
Human Implications and Concluding Remarks
The current observations demonstrate that trait levels of impulsive choice predict extinction resistance and the propensity to relapse, whereas transient changes in impulsivity do not correlate with relapse vulnerability. These observations are in line with the finding that ADHD and drug dependence often co-occur (Lynskey and Hall, 2001), and that methylphenidate, improving clinical signs of ADHD, does not concurrently reduce drug craving or relapse to cocaine use (Schubiner et al, 2002). The differences observed between trait impulsive choice and the acute treatment of impulsive choice further emphasize the importance of investigating the treatment potential of risk factors for drug dependence. Furthermore, these data suggest that rehabilitation programs aimed at acute, short-term, reduction of impulsivity will not suffice to maintain treatment retention.
References
Anker JJ, Perry JL, Gliddon LA, Carroll ME (2009). Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav 93: 343–348.
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science 320: 1352–1355.
Blondeau C, Dellu-Hagedorn F (2007). Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry 61: 1340–1350.
Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y (2005). Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol 526: 36–50.
Castells X, Casas M, Perez-Mana C, Roncero C, Vidal X, Capella D (2010). Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev (2). Art. No. CD007380.
Chamberlain SR, Del CN, Dowson J, Muller U, Clark L, Robbins TW et al (2007). Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62: 977–984.
Cheung TH, Cardinal RN (2005). Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci 6: 36.
Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003). Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol 11: 18–25.
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K et al (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–1270.
Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW (2005a). Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 182: 579–587.
Dalley JW, Theobald DE, Berry D, Milstein JA, Laane K, Everitt BJ et al (2005b). Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology 30: 525–537.
De Wit H (2009). Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14: 22–31.
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, De Vries W, Schoffelmeer AN et al (2008). Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63: 301–308.
Economidou D, Dalley JW, Everitt BJ (2011). Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry 69: 266–274.
Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ (2009). High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry 65: 851–856.
Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW (2010). Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biological Psychiatry 68: 770–773.
Evenden JL (1999). Varieties of impulsivity. Psychopharmacology (Berl) 146: 348–361.
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH et al (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30: 296–309.
Fuchs RA, Feltenstein MW, See RE (2006). The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci 23: 2809–2813.
Gipson CD, Bardo MT (2009). Extended access to amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology (Berl) 207: 391–400.
Goldstein RZ, Volkow ND (2011). Oral methylphenidate normalizes cingulate activity and decreases impulsivity in cocaine addiction during an emotionally salient cognitive task. Neuropsychopharmacology 36: 366–367.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412: 141–142.
Grimm JW, Shaham Y, Hope BT (2002). Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13: 379–388.
Heil SH, Johnson MW, Higgins ST, Bickel WK (2006). Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav 31: 1290–1294.
Jacobs EH, Smit AB, De Vries TJ, Schoffelmeer ANM (2003). Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24: 566–573.
Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146: 373–390.
Kirby KN, Petry NM (2004). Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 99: 461–471.
Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A et al (2007). Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend 88: 79–82.
Li CSR, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JLK et al (2010). Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci USA 107: 14455–14459.
Lynskey MT, Hall W (2001). Attention deficit hyperactivity disorder and substance use disorders: is there a causal link? Addiction 96: 815–822.
Mazur J (1987). An adjusting procedure for studying delayed reinforcement. In: Commons M, Mazur J, Nevin J, Rachlin H (eds). Quantitative Analysis of Behavior, vol V: The Effect of Delay and Intervening Events. Erlbaum: Hillsdale.
Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ et al (2010). Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci 124: 470–477.
Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z et al (2008). Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry 32: 34–41.
Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA (2009). Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci 123: 382–396.
Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM et al (2006). Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry 45: 468–475.
Paine TA, Dringenberg HC, Olmstead MC (2003). Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res 147: 135–147.
Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK et al (2002). Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22: 2916–2925.
Paterson NE, Ricciardi J, Wetzler C, Hanania T (2011). Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and D-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res 69: 41–50.
Pattij T, Vanderschuren LJ (2008). The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29: 192–199.
Perry JL, Carroll ME (2008). The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 200: 1–26.
Perry JL, Nelson SE, Carroll ME (2008). Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol 16: 165–177.
Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X et al (2008). Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33: 1028–1037.
Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G (2007). Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci 27: 245–250.
Schenk S, Partridge B (1999). Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology 147: 285–290.
Schippers MC, Schoffelmeer ANM, Pattij T, De Vries TJ (2011). Impulsive decision making does not predict vulnerability to heroin seeking and taking. Eur Neuropsychopharmacol 21: S38.
Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N et al (2002). Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol 10: 286–294.
Setlow B, Mendez IA, Mitchell MR, Simon NW (2009). Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav Pharmacol 20: 380–389.
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20.
Shepard JD, Bossert JM, Liu SY, Shaham Y (2004). The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55: 1082–1089.
Simon NW, Mendez IA, Setlow B (2007). Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci 121: 543–549.
Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M et al (2003). Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry 160: 1078–1085.
van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ (2006a). Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 187: 73–85.
van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ (2006b). Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry 60: 66–73.
Verdejo-Garcia A, Perez-Garcia M (2007). Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology 190: 517–530.
Wilens TE, Adler LA, Weiss MD, Michelson D, Ramsey JL, Moore RJ et al (2008). Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 96: 145–154.
Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A et al (2009). Increased impulsivity during withdrawal from cocaine self-administration: role for {Delta}FosB in the orbitofrontal cortex. Cereb Cortex 19: 435–444.
Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW (2003). Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 167: 304–314.
Winstanley CA, Eagle DM, Robbins TW (2006). Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26: 379–395.
Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI et al (2007). DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci 27: 10497–10507.
Winstanley CA, Olausson P, Taylor JR, Jentsch JD (2010). Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res 34: 1306–1318.
Winstanley CA, Theobald DE, Cardinal RN, Robbins TW (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24: 4718–4722.
Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM et al (2006). Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev 77: 1016–1033.
Zeeb FD, Floresco SB, Winstanley CA (2010). Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 211: 87–98.
Acknowledgements
We thank Rob Binnekade, Mathijs Stegeman, Yvar van Mourik, and Dustin Schetters for excellent technical assistance. This study was financially supported by a grant from the Netherlands Organization for Scientific Research (ZonMW grant no. 31160003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting potential conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Broos, N., Diergaarde, L., Schoffelmeer, A. et al. Trait Impulsive Choice Predicts Resistance to Extinction and Propensity to Relapse to Cocaine Seeking: A Bidirectional Investigation. Neuropsychopharmacol 37, 1377–1386 (2012). https://doi.org/10.1038/npp.2011.323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.323
Keywords
This article is cited by
-
Dissociable effects of cocaine and yohimbine on impulsive action and relapse to cocaine seeking
Psychopharmacology (2017)
-
Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone
Psychopharmacology (2016)
-
Dopaminergic function and intertemporal choice
Translational Psychiatry (2015)
-
Effects of the combination of wheel running and atomoxetine on cue- and cocaine-primed reinstatement in rats selected for high or low impulsivity
Psychopharmacology (2015)
-
Discrepant effects of acute cocaine on impulsive choice (delay discounting) in female rats during an increasing- and adjusting-delay procedure
Psychopharmacology (2015)